Introduction
- Available evidence suggests that the lymphoid system consists of two compartments, as shown in Figure 1:
- A central compartment in which stem cells originating in the bone marrow proliferate and differentiate in the bone marrow and thymus independent of antigen contact (i.e., antigen-independent differentiation)
- A peripheral compartment consisting of lymph nodes, spleen, and MALT
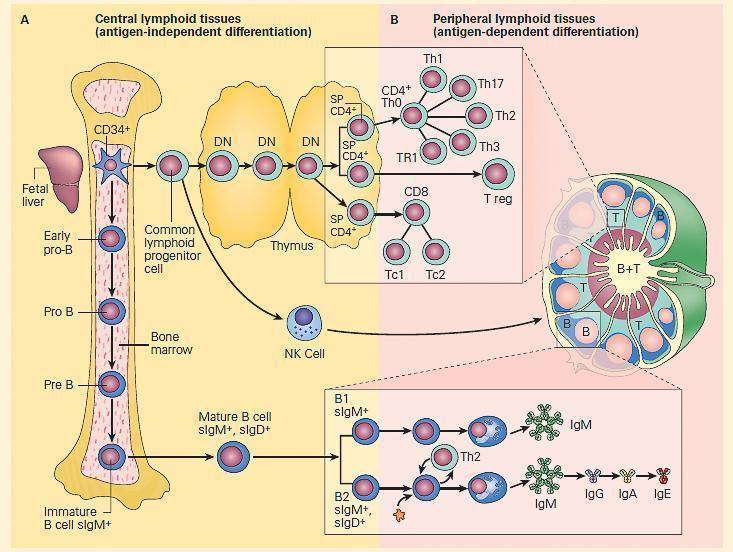
Figure 1. Schematic representation of the two compartments of lymphoid differentiation: central and peripheral compartments. Panel A: Development of the immune system from stem cells originating in bone marrow, fetal yolk sac or liver, and differentiating in central lymphoid tissues, i.e., bone marrow and thymus independent of antigen contact. Panel B: Migration of cells into peripheral lymphoid tissues in lymph nodes, spleen, and mucosa-associated lymphoid tissues at sites where these cells can react with antigen (i.e., antigen-dependent differentiation). B cells migrate to outer regions of lymph nodes in germinal centers; T cells migrate in inner paracortical areas; B and T cells are found in medullary cords. The insets show the location of the various subsets of T cells in the paracortical areas (upper inset) and B cells in the germinal centers of a lymph node. B cells respond to polysaccharides with the production of IgM antibodies, and B2 lymphocytes respond to protein antigens and with the help of Th2 lymphocytes lead to the sequential production of IgM, IgG, IgA, and IgE antibody. [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
Hematopoiesis Occurs in Human Bone Marrow
- The differentiation of cells destined to perform innate and acquired immune functions in the developing human have a common ancestral origin with cells of the hematopoietic system (Figure 2).
- Both cell types destined for hematopoiesis or lymphopoiesis appear to arise from a common population of pluripotent CD34+ hematopoietic stem cells (HSCs) of the bone marrow.
- Depending upon the type of microchemical environment surrounding the cells, development will occur along two avenues: the myelocytic and the lymphocytic (Figure 2).
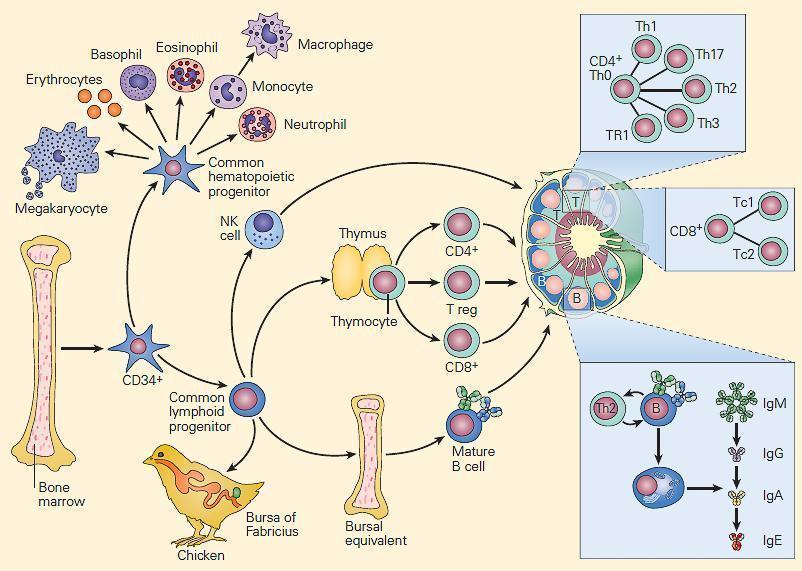
Figure 2. Schematic representation of the ontogeny of the immune system showing differentiation of progenitor cells into hematopoietic and immunocompetent lymphopoietic cells from a common population of pluripotent CD34+ hematopoietic stem cells of the bone marrow. Myeloid precursors differentiate into erythroid, megakaryocytic, and granulocytic/monocytic lineages, whereas lymphoid precursors develop into NK, T, and B cells. The common lymphoid progenitor cells can differentiate along two additional pathways. T cell development requires the influence of the thymus, while B cells develop in the microenvironment of the bursal-equivalent, the bone marrow in the human. Following differentiation, T and B cells populate distinct T and B cell regions in lymph nodes, respectively. [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
- All hematopoietic cells differentiate into mature cells with the aid of soluble mediators (i.e., cytokines) and contact signals from stromal cells, through various intermediate cell types that are defined by expression of cell surface antigens (see Chapter 9, Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012).
- The first step in hematopoietic development is differentiation of HSCs into myeloid and lymphocyte precursors.
- Myeloid precursors differentiate into erythroid, megakaryocytic, and granulocytic/monocytic (GM) lineages, whereas lymphoid precursors develop into NK, T, and B cells (Figure 2).
- The lymphoid precursors can differentiate along two additional pathways.
- T cell development requires the influence of the thymus, while B cells develop in the microenvironment of the bursal-equivalent, the bone marrow in the human from the common lymphoid progenitor (CLP) (Figure 2).
- In response to danger signals produced when the host encounters a foreign invader, e.g., microbes, allergens, transplants, or tumor cells, cytokines and chemokines are released.
- These cause many of the circulating leukocytes to migrate from the bone marrow into the blood and from the blood into the tissues, where they can remove these foreign agents that induce inflammation and can begin to repair the damaged tissues (see Chapter 5, Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012).
- It should be noted that this is a dynamic process with leukocytes moving between lymphoid organs, tissues (non-lymphoid) and the blood circulation in response to chemokines and cytokines and antigen (in the case of lymphocytes)
- Most of the blood leukocytes that emigrate into tissues are end-stage cells of development and do not replicate, e.g., neutrophils and eosinophils, with the notable exception of monocytes, which, after migration, differentiate into tissue macrophages.
- When present in specialized microenvironments in tissues, the pluripotent CD34+ hematopoietic stem cells can differentiate into various other tissue-specific cells such as hepatocytes, neurons, muscle cells, or endothelial cells.
- However, the signals that regulate this differentiation are unknown.
- The HSCs also circulate in small numbers in the peripheral blood and can be harvested as a source of progenitor cells for use in the reconstitution of patients receiving bone marrow transplantation (see Chapter 20, Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012).
Production of Specific T and B Lymphocytes Occurs in Antigen-Independent and Antigen-Dependent Phases
- T and B lymphocytes are characterized and defined by their randomly generated and vastly diverse antigen receptors, i.e., the T cell receptor (TCR) and the B cell receptor (BCR)
- These receptors are generated in each developing lymphocyte through a process called somatic recombination, in which enzymes encoded by recombination-activating genes (RAGs) splice gene segments to generate unique variable (antigen-binding) regions of the BCR (immunoglobulin) and TCR molecules (see Chapter 6 and 7, Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012)).
- This process is unique to lymphocyte development, and from a few hundred available gene segments can generate large numbers (potentially 1015 to 1018) of different antigen specificities.
- Somatic recombination occurs in the bone marrow (B cells) and thymus (T cells) in the absence of antigen, i.e., the antigen-independent phase.
- If a B cell or T cell then encounters its specific antigen in the peripheral lymphoid tissue (along with other appropriate stimuli), the cell proliferates and differentiates into a clone of specific effector cells (antibody-secreting plasma cells, cytotoxic T cells, or helper T cells)
- Memory cells specific for the same antigen are also produced, i.e., the antigen-dependent phase.
- Although the ancestral home of the lymphocyte precursor cell for both T and B cells is the bone marrow, T cells carry out their maturation in the thymus, in contrast to B cells, which continue their development in the bone marrow.
- Figure 3 displays a summary representation of both the antigen-independent central sites of differentiation for T, B, and NK lymphocytes and their subsequent antigen-dependent sites of differentiation in peripheral tissues showing the various stages of maturation that these cellular lineages undergo.
- The state of maturation of the cells undergoing progressive development is characterized by the acquisition of cell surface antigen receptors and molecules referred to as the cluster of differentiation (CD) molecules.
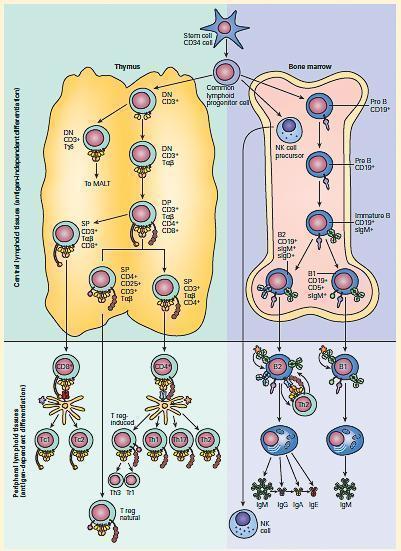
Figure 3. Schematic representation of the antigen-independent central sites of differentiation for T, B, and NK lymphocytes in the thymus and bone marrow and their subsequent antigen-dependent sites of differentiation in peripheral lymphoid tissues. [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
T Cell Development Originates in the Thymus – Functional Effector Cells and Memory Cells Are Produced in Peripheral Lymphoid Tissues
- T cells are identified by the presence of CD3, a signal transduction molecule that is expressed with TCR, and the lineage specific CD4 (T helper) or CD8 (T cytotoxic) markers.
- Most T cells express a TCR composed of one alpha (α) and one beta (β) chain (αβTCR), each of which has a V and a C region, and which together resemble one ‘‘arm’’ of the immunoglobulin Y-shaped molecule.
- A small minority of T cells have a TCR composed of one gamma (γ) and one delta (δ) chain (γδTCR) (Figure 3).
- Following migration of CD34+ stem cells from the bone marrow to the thymus, a series of intrathymic maturational steps occurs
- This includes somatic recombination of the TCR genes, that result in the transformation of a CD3+, CD4— CD8— double negative (DN) cell into a major population of CD3+ CD4+, CD8+ double positive (DP) αβTCR-expressing T cells and a minor population of CD3+ CD4—, CD8— DN γδT cells.
- The γδT cells ultimately function as intraepithelial lymphocytes (IEL) at mucosal sites (Figure 3).
- The CD3+ CD4+, CD8+ DP population further differentiates into three
- (1) a CD3+, CD4—, CD8+ single positive (SP) T cell that migrates to the peripheral tissues as a CD8+ T cytotoxic (Tc) population;
- (2) a CD3+, CD4+, CD25+ SP T cell that migrates to the peripheral tissues as a T regulatory (Treg) population; and
- (3) a ‘ CD3+, CD4+, CD8— SP T cell that migrates to the peripheral tissues as a CD4+ T helper (Th) population.
- The transformation of these precursor cells into Tc, Treg, and Th subpopulations each involve signals provided by cytokines and by binding to either MHC-I or MHC-II on thymus epithelial cells.
- T cells that have generated self-specific TCR undergo apoptosis in the thymus.
- In the peripheral lymphoid tissues, the CD8+ Tc cells interact with antigen processed by APCs, e.g., DCs, by the endogenous pathway and presented on MHC-I.
- Tc can further differentiate into T cytotoxic subpopulations, e.g., Tc1, Tc2, Th17, and others based on their cytokine secretion.
- The CD4+ Th cells interact with antigen processed by APCs by the exogenous pathway and presented on MHC-II.
- Th can further differentiate into Th1, Th2, Th17, and Treg (induced) subpopulations.
- The CD3+, CD4+, CD25+ SP T cell ultimately migrates to the peripheral tissues and functions as a ‘‘natural’’ Treg natural population.
B Cell Development Begins in the Bone Marrow – Functional Effector Cells and Memory Cells Are Produced in Peripheral Lymphoid Tissues
- The CD34+ stem cells in the bone marrow destined for B cell production go through a number of differentiative steps.
- Contact with bone marrow stromal cells is necessary to supply the correct environmental signals for the pro-B cell to further differentiate.
- B cells initially need direct contact with stromal cells (VLA-4 on pro-B cells and VCAM-1 on stromal cells).
- After initial contact, a receptor on pro-B cells encoded by c-Kit, called KIT (a tyrosine kinase) interacts with a stromal cell molecule called stem-cell factor; c-Kit is then activated, causing the B cell to divide and to express receptors for IL-7.
- The production of IL-7 will eventually down-regulate the adhesion molecules, and the B cell is released still requiring IL-7 for growth and maturation.
- Somatic recombination of gene segments encoding the heavy chain variable region commit the progenitor cell to the B cell lineage and it becomes a pre-B cell;
- Expression of membrane IgM (sIgM) defines the immature B cell.
- Immature B cells that bind self-antigen undergo apoptosis in the bone marrow and are deleted.
- Those that survive, differentiate into two subpopulations:
- (1) a B1 CD19+ CD5+ sIgM+ cell that migrates into the peripheral lymphoid organs as a B1 cell;
- (2) a B2 CD19+ sIgM+ surface IgD (sIgD+) cell that migrates into the peripheral lymphoid organs as a B2 cell (Figure 1).
Natural Killer Cells Also Develop in the Bone Marrow
- Although usually assigned to the innate immune system, NK cells develop from CD34+ stem cells in bone marrow and account for up to 15 percent of peripheral blood lymphocytes.
- Therefore, they are included in the description of the ontogeny of the adaptive immune system together with T and B cells (Figure 2).
- NK cells do not have antigen-specific receptors; they have receptors that bind self carbohydrates onto host cells to activate NK killing and other receptors that recognize self MHC alleles to inhibit the killing of normal cells.
- Morphologically, NK cells have the appearance of large granular lymphocytes with neither T cell nor B cell antigen receptors.
- NK cells do express CD16 (an Fc receptor for IgG) and CD56 (an NK-specific adhesion molecule), important markers for the identification of NK cells in the peripheral circulation.
- NK cells are involved in a variety of immune reactions in the recognition and killing of target cells utilizing direct and antibody-dependent cellular cytotoxicity (ADCC) mechanisms.
The Mature Immune System
- Figure 4 shows the distribution and functions of different cells of the fully developed immune system
- Immunity is in a homeostatic balance and the different anatomical compartments facilitate this balance: between events at mucosal surfaces and within primary and secondary lymphoid organs
- Different infectious agents offset this balance and the immunity is perpetually in a state of regulation between tolerance to self and responding to non-self antigen
- Some individuals are able to mount immune responses which leads to immune control of infectious agents, such as to malaria, TB and HIV
- The mechanisms behind such immune control may not always be clear or known, but having an intact immune system is a key attribute of immune control.
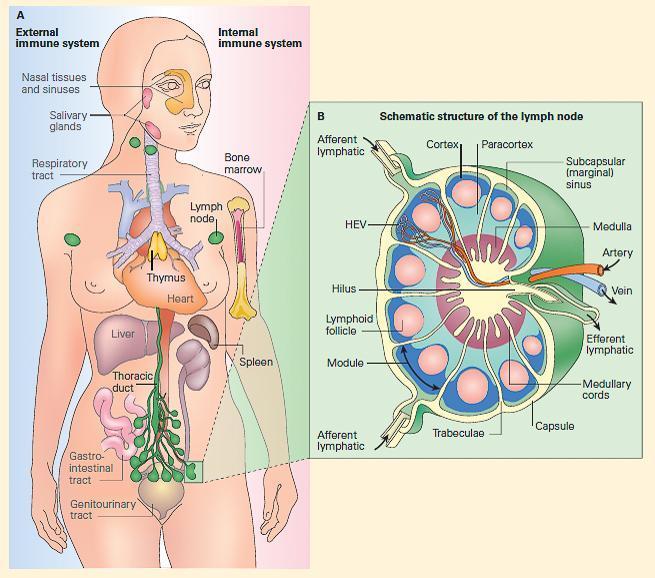
Figure 4. Panel A: Schematic representation of the fully developed immune system in the human in the external immune, or MALT, or the internal immune system; both consist of a network of lymphoid tissues composed of macrophages, dendritic cells, and T and B lymphocytes organized either as loose collections of these cells in lymphoid follicles or as more highly organized organs such as lymph nodes and spleen. Panel B: Schematic structure of a lymph node showing its organization into a cortex, paracortex, and medulla with primary and secondary follicles in the cortex and medullary cords in the medulla and a lymphatic supply (entering through afferent vessels in the cortex and exiting through a single efferent lymphatic vessel in the hilus) and an afferent and efferent blood supply entering and leaving through the hilus.










