Introduction
- The T cell repertoire in a healthy adult is shaped by thymic selection (positive and negative), where naive CD4+ and CD8+ T cells can then interact, and be primed, with “foreign” antigen in the secondary lymphoid tissues
- Antigen engagement via the T cell receptor (TCR) then shapes the repertoire of antigen-specific T cells and most likely the functional attributes of the T cell
- Multiple phenotypes of both CD4+ and CD8+ T cells have been identified which have differing functions
- The key to understanding T cell immunity is knowing the types of T cells and how they expand and contract with antigen and how this process is regulated
- In this section, we will deal with Treg cells and how immunity to microbes is regulated and suppressed
Lymphocytes Perform Adaptive Immune Functions
- The cells of the adaptive immune system, in contrast to those of the innate immune system, interact with the environmental agent in a highly discriminative way, i.e., they display specificity, heterogeneity, and memory.
- These functions are primarily carried out by two types of cells that are involved in the recognition of antigen:
- (1) the thymus-dependent or T lymphocytes, which participate in cellular responses against intracellular pathogens, organ transplants, and malignant cells
- (2) the bone marrow or bursal-dependent B lymphocytes, which provide humoral immunity, i.e., antibody-mediated immunity against extracellular pathogens, their toxins, and other environmental substances.
- As outlined in the previous section, a third group of cells involved in the presentation of antigen to T cells, i.e., APCs, include dendritic cells, macrophages, and B cells (Table 1-8).
- APCs take up predominantly protein antigens, cut them into pepti-des, bind the peptides to major histocompatibility complex (MHC) molecules, and display these presented antigens on their cell surface, where they can be recognized and bound by antigen receptors on T lymphocytes.
- T lymphocytes are identified by a surface cluster of differentiation (CD) molecule named CD3 and are comprised of two major groups: the CD4 and CD8 populations
- The CD4 cells display helper activities on other populations of cells, and in turn are subdivided into at least Th1, Th2, Th9, Th17 and T regulatory (Treg) groups, each with a characteristic profile of production cytokines.
- The CD8 T cytotoxic population is the second major group of T lymphocytes that function in killing target cells; they are comprised of Tc1 and Tc2 subpopulations with similar cytokine profiles as Th1 and Th2 cells.
- Collectively, the T lymphocytes play or facilitate a central role in the orchestration of all functions of the adaptive immune system and perform four important tasks:
- (1) promotion of inflammation by cytokine production (Th1 and Th17 cells)
- (2) helping B lymphocytes (Th2 cells)
- (3) regulating immunosuppressive responses (T regulatory cells)
- (4) killing of unwanted target cells (CTL)
How T cells “see” Antigen
- Because the role of T lymphocytes is to deal with intracellular infections and ‘‘altered self’’ cells (tumor cells), they must have a way to recognize intracellular antigen.
- In addition to their role in innate immunity, dendritic cells and macrophages also play a major collaborative role in the presentation of antigen to T lymphocytes of the adaptive immune system and are therefore referred to as APCs (Table 1).
- Following the uptake and digestion by APCs, foreign substances, usually proteins, are processed by proteolysis into peptide fragments that are later presented to T cells in a highly discriminative manner.
- This process employs cell receptors consisting of molecules on both the surface of the APC membrane (i.e., MHC proteins) (see Chapter 10, Bellanti JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012 and the previous section) as well as a specific antigen-binding receptor on the T cell membrane, the TCR
- Of these, the dendritic cells are the most potent APCs, and are particularly important in initiation and promotion of subsequent adaptive immune responses.
- In addition to these cells of the innate immune system, B cells of the adaptive immune system, as described below, can also serve as APCs
Table 1. Various types of antigen-presenting cells
| Type | Location |
|---|---|
| Macrophages | Widely dispersed in tissues |
| Alveolar macrophages | Lung |
| Langerhans cells | Skin |
| Kupffer cells | Liver |
| Microglial cells | Central nervous system |
| Dendritic cells | Widely dispersed in tissues |
| B lymphocytes | Lymph nodes and other lymphoid tissues |
Antigen Processing and Presentation Follows Different Pathways for Cytosolic (Endogenous) and Vesicular (Exogenous) Antigen
- T lymphocytes play a pivotal role in both cell-mediated and humoral immune responses of the adaptive immune system.
- These functions are carried out by T lymphocytes that interact with antigen through the TCR.
- The processing of antigen can occur at two sites: (1) at the level of APCs or (2) at the target cell site.
- Phagocytes and other APCs play major roles in internalizing, processing, and presentation of ‘‘processed antigen’’ to T lymphocytes for induction of immune responses carried out by both CD4 and CD8 lymphocyte populations.
- Antigen can also be processed and presented to T lymphocytes at the target cell site in a cell that has been infected with a virus, for example, or modified by a chemical or by malignant transformation.
- Shown in Figure 1 is a schematic representation of the two modes of antigen processing at these two sites that determines which MHC the processed antigen will react with.
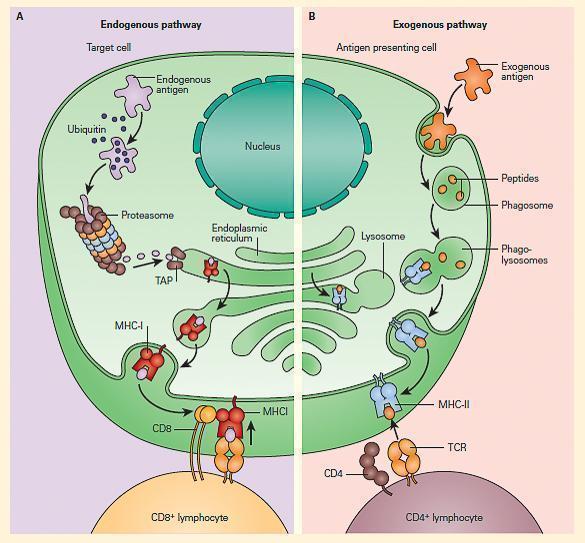
Figure 1. Schematic representation of the two modes of antigen processing. Panel A: Shows the endogenous pathway, which presents processed antigen (i.e., peptides) from a target cell to a CD8þ lymphocyte in the context of MHC-I. Panel B: Shows the exogenous pathway, which presents the peptide products from an APC to a CD4+ lymphocyte in the context of MHC-II. [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
- In the case of antigens processed by APCs by the exogenous pathway, CD4 T cells recognize antigen that has been processed into peptide fragments (epitopes) that are then placed in a groove of the MHC-II molecule, and presented to the TCR on subsets of helper T cells (called CD4+ cells).
- Other antigens found within cells, e.g., target cells, are processed through an endogenous pathway and are delivered by MHC-I to TCR of ‘‘cytotoxic’’ T cells (CD8+ cells).
The Interaction Between APC and T Cells Influences Which T Cells Are Activated
- CD4+ T cell activation results in the secretion of cytokines that help and regulate other cells (see Chapter 9, Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012).
- The pattern of cytokine expression defines the subsets of CD4+ T cells: Th1, Th2, Treg1, Th3, and Th17 cells.
- Th1 cells secrete interferon gamma (IFN-y) and create a milieu in which key cytotoxic effectors—macrophages, natural killer cells, and cytotoxic CD8+ T lymphocytes—are activated, generating cell-mediated immunity.
- Th2 cells secrete IL-4 and IL-10 (and other cytokines) and help antigen-primed B lymphocytes differentiate into plasma cells and secrete antibodies, the effector molecules of humoral responses.
- T cells, Treg cells, with the phenotype CD4+CD25+, express the signature transcription factor FOXP3 and usually secrete IL-10 and transforming growth factor beta (TGF-B).
- Cells with this phenotype are thought to recognize self-antigens and function to prevent autoimmunity and are also involved in chronic viral infections, allergy, transplantation, and malignancy.
- Th17 cells represent a wide variety of recently described cells involved in inflammation through the elaboration of proinflammatory cytokines and interact with IL-23 (see Chapter 9, Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012).
- As described in the previous section, T cells can recognize peptide fragments that have been processed and presented by APC, i.e., dendritic cells (DC), macrophages, and B cells.
- Figure 1-21 depicts the shaping of T cell subsets after interacting with antigen and the polarization of T cells in response to different cytokines
- Production of Th1, Th2, Th17, and two populations of Treg cells Th3 and TR1 (Figure 2), which have a variety of interactions with other cells in performing the following functions:
- promotion of inflammation by cytokine production (Th1 lymphocytes);
- helping B lymphocytes (Th2 lymphocytes);
- regulating immunosuppressive responses (Treg lymphocytes).
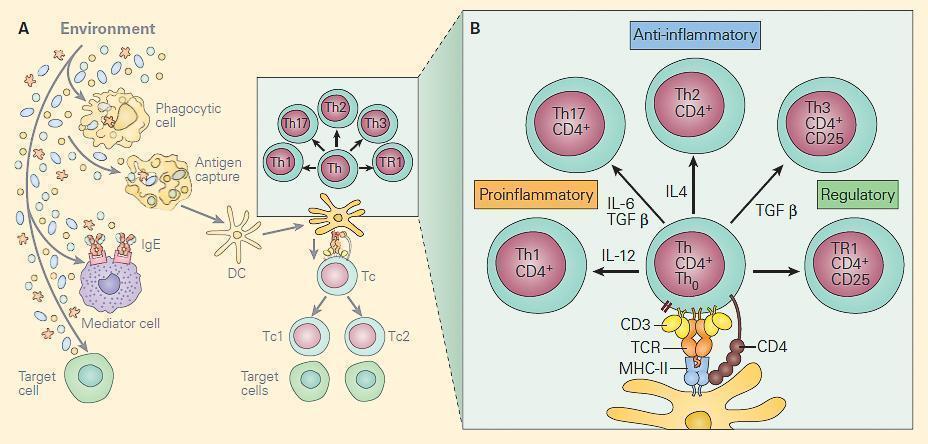
Figure 2. Panel A: The two main T cell populations are CD4+ and CD8+ cells. The CD4 are helper T cells and are shown highlighted with the CD4+ subsets Th1, Th17, Th2, Th3, and Tr1 and shown below are the CD8 cytotoxic T cells (faded). Panel B: Shows the molecular events in the immunologic synapse at the CD4+/dendritic cell interface together with the cytokines that induce the Th0 differentiation into each of the subsets. [Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
Cytokines that Determine T-cell Subsets
- Specific cytokines are involved in shaping the two subsets of the T-cell system: CD4+ Thelper (Th) and CD8+ Cytotoxic T Lymphocytes (CTL)
- The Th population has several effector subsets (Figure 1), including Th1, Th2, Th17, and Treg, which have roles in delayed hypersensitivity, antibody production, inflammation, and immunosuppression (or regulation), respectively
- Th are the key orchestrators of adaptive immunity in mammals, and each effector subset mobilises a distinct module of antimicrobial immunity:
- Th direct elimination of intracellular microbial pathogens and tumors
- Th2 induce expulsion of helminths
- Th17 promotes resistance to extracellular bacteria and fungi, playing important roles in protection at mucosal surfaces and in promotion of autoimmune inflammation, as well as participating in antitumor immune responses
- Treg suppress inflammatory reactions and ensure host autoreactive lymphocytes do not mount a response against host tissues or innocuous environmental antigens
- CTL have Tc1 and Tc2 subsets: Tc1 destroy virally-infected or malignant cells (Figure 3)
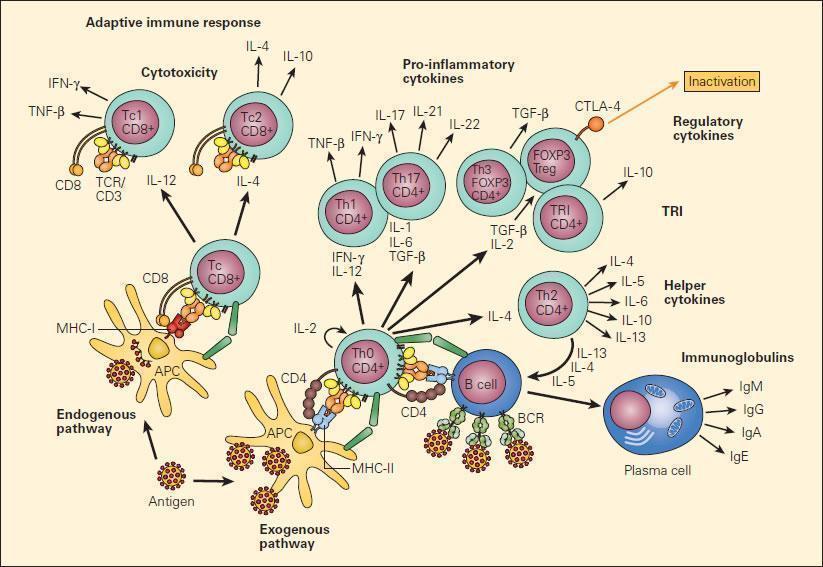
Figure 3. Schematic representation of the two major pathways of T cell differentiation: the Th and Tc populations and their subsets. Following uptake and processing of an antigen by an APC shown in the figure as a dendritic cell, peptide is presented either to the CD8 population in the context of MHC-I or to the CD4 subpopulation in the context of MHC-II following which a cascading set of cellular lymphoproliferative and differentiative steps are initiated under the inductive influence of cytokines that ultimately determine their effector functions [Reproduced with permission from Bellanti JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012].
T cell Classification
| T-cell type | T-cell receptor type | Glycoprotein coreceptor | Antigen-presenting molecule | Foreign stimulus | Function or defining characteristics |
|---|---|---|---|---|---|
| Naive | Any | Any | Any | Any | Has not encountered an antigen |
| Th1 | αβ | CD4 | MHC II | Virus/intracellular bacteria | Activates macrophages; causes other cells to go on guard against a virus, quarantining it |
| Th2 | αβ | CD4 | MHC II | Parasites | Stimulates eosinophils, basophils, and mast cells to eliminate parasite; stimulates B cells to produce IgE and IgA antibodies |
| Th9 | αβ | CD4 | MHC II | Parasites | Supports CD4 + T-cell expansion and survival; recruits mast cells |
| Th17 | αβ | CD4 | MHC II | Extracellular bacteria/fungi | Recruits neutrophils, which kill many bacteria and fungi |
| T-follicular helper (Tfh) | αβ | CD4 | MHC II | Any | In follicles of spleen and tonsils, stimulates B-cell production of high-affinity antibodies |
| Regulatory T cell (Treg) | αβ | CD4 | MHC II | NA | Regulates T-cell activation and proliferation |
| Cytotoxic T lymphocyte (CTL) | αβ | CD8 | MHC I | Any | Releases vesicles containing perforin, which punctures the target cell, and granzyme, which induces apoptosis, into the vicinity of infected cells, destroying them |
| Central memory (Tcm) | αβ | CD4 or CD8 | MHC II or MHC I, respectively | Any | Responds to secondary infections by proliferating; also circulates in blood, peripheral organs, and lymphoid organs, fighting secondary infections, but less so than Tem; that is, focuses on proliferating |
| Effector memory (Tem) | αβ | CD4 or CD8 | MHC II or MHC I, respectively | Any | Travels around in tissues fighting secondary infections; also circulates through blood supply but avoids lymphoid organs (spleen, lymph nodes, lymphatic vessels); less proliferative than Tcm |
| Tissue-resident memory (Trm) | αβ | CD4 or CD8 | MHC II or MHC I, respectively | Any | Stays in the tissue where it previously fought an infection and fights secondary infections there; does not recirculate in blood or revisit lymphoid organs |
| Virtual memory | αβ | CD8 | Responds to cytokines, not antigens | Any | Antigen-inexperienced cell that leaves the thymus and becomes an activated memory cell without first encountering an antigen; cytokines can activate this cell type; particularly important early in life, when immune system has not seen many antigens, and late in life, when it is weakened |
| Innate memory | αβ | CD8 | Responds to cytokines, not antigens | Any | Antigen-inexperienced memory cell that develops and becomes activated in the thymus |
| Memory stem cell (Tscm) | αβ | CD4 or CD8 | MHC II or MHC I, respectively | MHC-restricted antigens | Stem cell–like progenitor of all other post-thymic T cells (both memory and effector) |
| γδ T cells | γδ | Usually double-negative (display neither CD8 nor CD4) | CD277 | Bacteria | In humans recognizes pyrophosphate intermediates of bacterial lipid synthesis; in humans, found in peripheral blood; may recognize other antigens, too (little is known about this cell type) |
Table 2. The function or defining characteristics of T cell types [Taylor, A.P. The Ever-Expanding T-Cell World: A Primer. The Scientist. August 7, 2017.]
B cell Interaction with CD4+ T cells
- The antigen primed B cells, expressing antigen-specific BCR’s, interacts with the Th2 CD4+ T cells (in the germinal centres) via MHC-II
- This cognate interaction is critical for B cells to differentiate to plasma cells
Watch video of T cell and B cell interactions
Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012.
Th1 Cells Signal Macrophages to Kill Engulfed Intracellular Bacterial Pathogens
- Mycobacterium tuberculosis is an example of a bacterium that can be phagocytosed by macrophages and is able to protect itself from being killed by virtue of its intracellular location within the phagolysosome.
- The organism can now replicate in the phagosome, protected from the harmful effects of the humoral antibody immune response.
- Th1 cells recognize peptides on the macrophage membrane in association with MHC-II, produce cytokines, and signal the macrophage with IFN-g together with other cytokines to kill the bacteria.
- Other Th1 cytokines also attract more macrophages to the infection site and activate them to produce inflammatory cytokines that result in delayed-type hypersensitivity, e.g., the TST.
- One outcome of the host-microbial interaction between the macrophage and the intracellular location of the tubercle bacillus is the killing of the tubercle bacillus resulting from enhanced macrophage killing of the bacillus by activation by IL-12 and later Th1 production of IFN-g
- Or there is failure of killing when the replication of the tubercle bacillus overwhelms the macrophage capacity.
Activated CD8 Cytotoxic T Lymphocytes kill target cells
- Cytotoxic CD8+ T cells effect their function by recognizing peptide bound to MHC-I
- The peptide has been endogenously processed either directly (derived from viral genes, such as HIV) or indirectly, through cross-presentation (derived from effete or dead bacterially infected cells, such as mTB).
- Figure 4 shows activated CD8+ cytotoxic T lymphocytes recognizing a bacterial peptide presented by HLA-B27 (derived from Immunopaedia case study: 14 year old with severe hip pain)
- This causes liberation of cytotoxic granules (perforin and granzymes) from the CD8+ T cell which then cause lysis and apoptosis of infected target cells
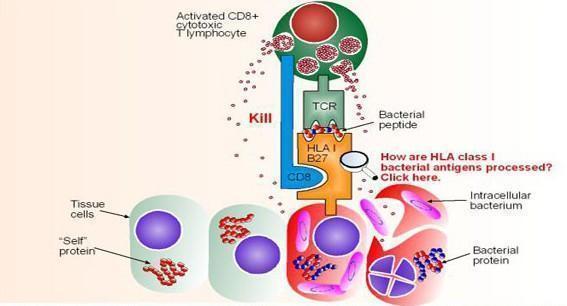
Figure 4. Recognition of a bacterial peptide by activated CD8+ cytotoxic T lymphocytes after cross-presentation and endogenous processing of the bacterial proteins. Killing is effected by the release of granzymes and perforin from the activated CD8 cell upon contact. [from Immunopaedia case study: 14 year old with severe hip pain].
Watch a video of the different killing mechanisms, including CD8 CTL
Reproduced with permission from Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012.
Quiz
Related Talk
Virginie Rozot, South African TB Vaccine Initiative – T Cell Subsets
References
- Bellanti JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012
- Taylor, A.P. The Ever-Expanding T-Cell World: A Primer. The Scientist. August 7, 2017.










