B Cell Activation
- Resting B cells become activated by antigen via the BCR and/or by microbiological side products (pathogen associated molecular patterns; PAMP) via their toll like receptors (TLR4, 7, 9 in mice) and start to proliferate.
- Protein antigens become internalized, digested and presented to T cells as peptides via MHCII.
- Cognate B cell / T cell interaction provides co-stimulation to B cells via CD40, which becomes activated on B cells via CD40 ligand (CD40L) expressed on T cells.
- T cells also provide cytokines to B cells that support their survival (IL-4), differentiation into plasma cells (IL-21) or class switch recombination.
- In mice, Th1 cytokines, such as IFNγ, typical for antiviral responses, elicit IgG2 isotypes, Th2 cytokines, such as IL-4, IL-5 and IL-13, typical for parasite infections, elicits IgG1 and IgE responses.
Precursors of plasma cells
- Resting B2 B cells (these are follicular (FO) and marginal zone (MZ) B cells) express membrane bound IgM, the B cell receptor (BCR) of the IgM type
- In mice and likely also in humans, another B cell population that is located primarily to the pleural cavities exists, the B1 B cells, a self-renewing population. B1 B cells secrete natural IgM in a T cell independent manner and have a limited repertoire. They differentiate rapidly into short lived plasma cells
- MZ B cells reside in the marginal zone that surrounds the follicles of secondary lymphoid organs and is directly connected to the vasculature. MZ B cells therefore respond to blood born antigens. They react more rapidly to PAMPs and differentiate rapidly into short lived plasma cells
- FO B cells express also membrane bound IgD
- FO B cells reside in the follicles of secondary lymphoid organs and are long lived. They are typically the B cells that interact with cognate T cells.
- Memory B cells express membrane bound Ig of the IgM, IgG or IgA type.
Activation of B cells
- Activation of B cells via the BCR and/or PAMPs elicits proliferation of B cells.
- PAMPs, for instance lipopolysaccharide, also elicit differentiation of B cells into short-lived plasma cells secreting low-affinity antibodies.
- MZ B cells are especially prone to rapidly differentiate into short-lived plasma cells but FO B cells can also differentiate into short- lived plasma cells.
- B cell differentiation into plasma cells is coupled to a certain number of cell divisions that are required to allow expression of transcription factors which terminate the B cell program and initiate the plasma cell program.
- T cell dependent activation of B cells supports the generation of memory B cells and long-lived plasma cells secreting high affinity antibodies. This process requires specific microanatomical structures in secondary lymphoid organs, the germinal centers, where class switch recombination and somatic hypermutation occur.
- The transcriptional network that is initiated in B cells in a proliferation dependent manner and fosters plasma cell differentiation is outlined in Fig. 1 (Taken from Nutt et al., Nature Reviews in Immunology, 2015).
- An essential transcription factor for plasma cell differentiation is Blimp-1. Many essential functions in plasma cells are under the control of Blimp-1.
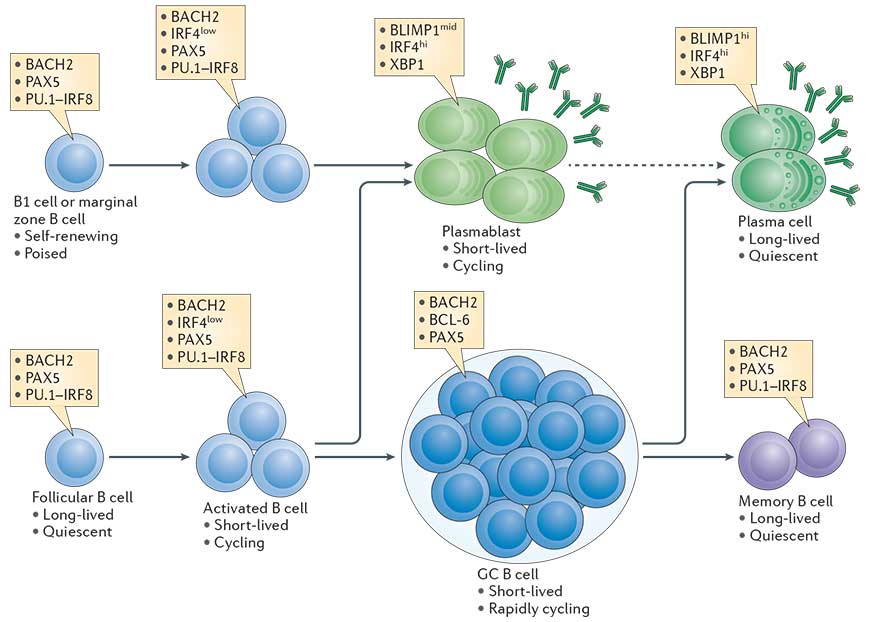
Figure 1: The cellular stages of late B cell differentiation. The majority of mature B cells are located in the follicles of lymphoid organs and are known as follicular B cells. Others specialized B cell subsets include marginal zone B cells, which localize to the region between the red and white pulp in the spleen, and B1 cells, which are found in the peritoneal and pleural cavities. All mature B cell subsets express the transcription factors paired box protein 5 (PAX5). PU.1 interferon-regulatory factor 8 (IRF8) and BTB and CNC homologue 2 (BACH2), whereas low levels of IRF4 are induced by antigen receptor signalling. B cells activated by antigen (also termed B lymphoblasts) are capable of rapid proliferation, immunoglobulin class-switch recombination, and differentiation into short-lived plasmablasts that express high levels of IRF4 and X-box-binding protein 1 (XBP1), and intermediate (mid) levels of B lymphocyte-induced maturation protein 1 (BLIMP1) and secreted antibody. Follicular B cells can also upregulate B cell lymphoma 6 (BCL-6) and repress IRF4 expression during the germinal centre (GC) reaction where affinity maturation of the antigen receptor occurs. B cells with high-affinity antigen receptors exit the GC and differentiate into either memory B cells, which express a similar transcriptional signature to mature B cells, or long-lived plasma cells, which express high levels of BLIMP1, IRF4 and XBP1 and produce large quantities of antibody. Although BLIMP1h plasma cells derive from BLIMP1mid cells, it remains unknown whether plasma cells are derived from plasmablasts (indicated by the dashed arrow) or from an earlier plasma cell-committed stage. The contribution of marginal zone B cells and B1 cells to the long-lived plasma cell compartment is also poorly characterized. [Nutt et al., Nature Reviews Immunology, 2015]
The germinal center (GC) reaction
- Germinal centers (GCs) are regions within secondary lymphoid organs which develop following T cell–dependent antigen exposure. These areas are sources of high-affinity antibodies.
- One of the fundamental pathways needed for the GC reaction is the interaction between activated follicular helper CD4 T (Tfh) cells and B cells.
- Tfh cells are essential for the GC reactiom as they play a role in increasing the efficiency of antibody responses
- GC Tfh cells expresse IL-21 at high levels. This cytokine is important for the differentiation and functioning of Tfh cells. These cells also produce IL-4, IFN-γ and IL-2 at moderate/variable levels. Bcl-6 is a transcription factor and an important marker of Tfh cells. This transcription factor is important for the development of Tfh and germincal center B cells.
- Tfh cells have been shown to play crucial roles in the production of high-affinity, long-lasting antibody responses to vaccines, autoimmune diseases, allergies and even cancer. These cells are important in HIV because they help in the development of broadly neutralising antibodies which can prevent entry of viruses across the different HIV subtypes.
- GC-committed B cells move to the center follicle due to loss of EBI2 and initiate the GC reaction.
- The GC reaction represents a checkpoint where the antigen-specific BCR repertoire is randomly altered in heavily proliferating GC B cells in the dark zone (DZ) through somatic hypermutation (SHM) induced by activation induced cytidine deaminase (AID).
- Mutated B cells having acquired affinity-enhancing mutations are selected by T cells in the light zone (LZ) of the GC or may migrate back into the DZ and undergo another round of SHM when their affinity is not competitive.
- Thus, B cells are positively selected through iterative cycles and elucidation of the underlying mechanisms will provide important information for vaccine development.
- The GC reaction is also required for class switch recombination (CSR). High-affinity plasma cells that migrate back into the BM are the endpoint of the GC reaction.
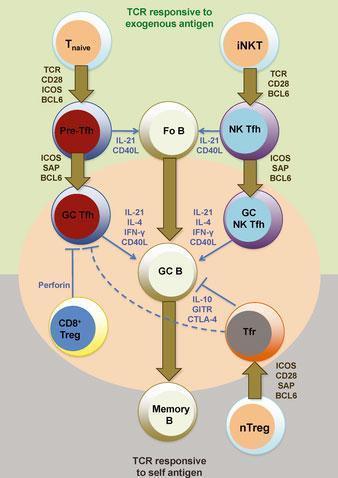
Figure 2: Heterogeneity and differentiation of germinal center T cells. The commitment of T cells in promoting germinal center B-cell responses against immunizing antigens is largely dependent at least initially on TCR engagement and CD28 costimulation by antigen-presenting dendritic cells. Upon transient BCL6 induction after activation, CD4+ naive T cells (Tnaive) give rise to pre-germinal center follicular helper T (pre-Tfh) cells that subsequently require contact with cognate B cells to receive re-enforcement signals via ICOS, PD1, and SAP signaling for BCL6 stabilization and commitment to the germinal center (GC) Tfh cell differentiation pathway. B-cell conjugation and SAP signaling is also important for NK Tfh cell formation from invariant NKT (iNKT) cells and their response toward glycolipid-containing antigens. T-cell-mediated suppression of autoreactive and/or or non-antigen-specific germinal center B cells is mediated by Qa-1-restricted CD8+ regulatory T (CD8+ Treg) cells (that directly inhibit Tfh cells) and follicular regulatory (Tfr) cells. Tfr cells develop from BCL6-dependent natural CD4+ regulatory T cells (nTregs) that receive TCR and CD28 signals and also and SAP-mediated signals during cognate B-cell interactions. Fo B, follicular B cells; GC B, germinal center B cell; memory B, memory B cell. [R, Ramiscal & C, Vinuesa. Immunological Reviews 2013 Vol.252: 146–155]
The plasma cell and antibody secretion
- Early plasma cells still proliferate and are also called plasmablasts or antibody secreting cells (ASC).
- The term “antibody secreting plasma cell” is unambiguous and refers to the terminally differentiated, non-proliferating plasma cell that secretes high amounts of antibody. Plasma cells are much larger than resting or even proliferating B cells.
- In plasma cells and plasmablasts, the exons coding for the membrane domain of Ig molecules of various isotypes have been removed and replaced by exons coding for sequences allowing antibody secretion.
- Plasma cells have a strongly expanded endoplasmic reticulum and express high amounts of molecules involved in antibody folding and secretion.
- Plasma cells can secrete several thousands of antibodies per second.
- Long-lived plasma cells migrate back to the bone marrow where their survival is supported in niches. Stable antibody titers upon vaccination have been observed for 20 years and more for some immunogens.
Quiz
Related Talk
Richard Koup, NIH – Germinal centres and tfh cells










