“The ancient Greeks, who performed sacrificial rites on very young and generally prepubertal animals, noted an extensive mass of tissue in the chest above the heart extending for some distance up into the neck and concluded that it must be the seat of the ´soul´.” Miller JAFP, Thymus 1:3-25, 1979
Video: https://youtu.be/NnMZ0Uxol-M
Jacopo Berengario da Carpi in his Isagoge breves made the first description of the thymus
William Hewson wrote ‘The thymus gland we consider as being an appendage to the lymphatic glands, for the more perfectly and expeditiously forming the central particles of the blood of the foetus, and in the early part of life. We have proved that vast numbers of central particles made by the thymus and lymphatic glands are poured into the blood vessels through the thoracic duct and if we examine the blood attentively we see them floating in it.’
In 1900, John Beard wrote “It has fallen to my lot to show that the first leukocytes arise in the thymus, from its epithelial cells, and that the thymus must be regarded as the parent source of all the lymphoid structures of the body…., so the original leukocytes, starting from their birth place in the thymus, have penetrated into almost every part of the body, and have created new centres for growth, for increase, and for useful work for themselves and for the body” Miller JFAP, Thymus 1:3-25, 1979
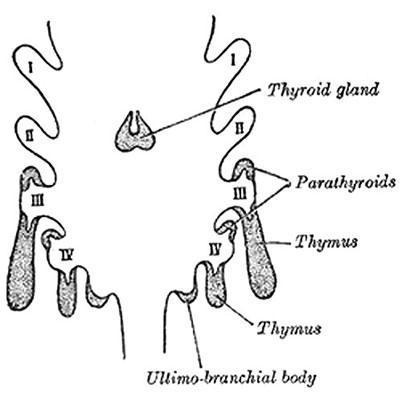
Weller reported that the thymus and the parathyroids are formed by the third and fourth endodermal pharyngeal pouches that migrate laterally in surrounding mesenchyme
Robert Good and Richard Varco described a new syndrome characterized by thymoma, lymphopenia, decreased serum gamma globulins and increased susceptibility to infections by encapsulated microorganisms, virus and fungi. The syndrome was known as Good´s syndrome
F. MacFarlane Burnet proposed in his “Clonal selection theory” that antigens are recognized by immunocompetent cells that either clonally expand to mount an immune response or are deleted resulting in tolerance. However, the identity of the immunocompetent cells was still unknown
In the late 1950s and early 1960s, James Gowans and colleagues in Oxford University demonstrated that lymphocytes obtained from the thoracic duct of rats and mice, labeled with 3H-thymidine and reinfused intravenously, were found in spleen and lymph nodes. Chronic drainage of thoracic duct resulted in depletion of small lymphocytes, unresponsiveness to different antigens and tolerance to skin homografts (allografts).
Gowans et al., Nature 196: 651-656, 1962; Gowans and Knight, Proc Roy Soc London B 159:257-282, 1964
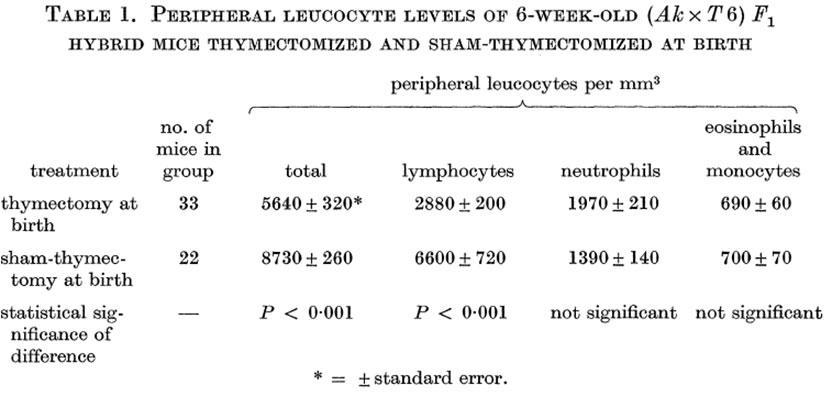
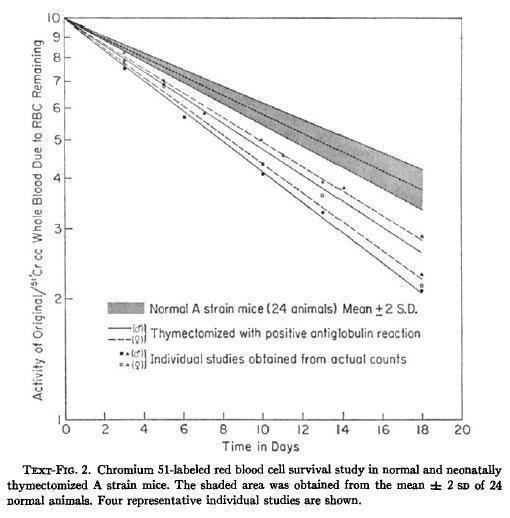
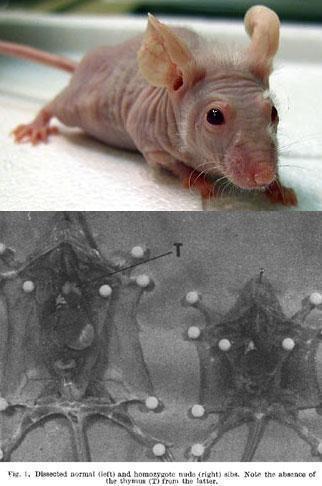
F. A P. Miller in a series of classical experiments demonstrated that neonatally thymectomized mice are lymphopenic and immunodeficient and proposed that the thymus is the lymphoid organ where small immunocompetent lymphocytes are differentiated.
Miller, Lancet 278:748-749, 1961; Miller, Nature 195: 138-1319, 1962; Miller, Proc Roy Soc London B 16:415-428, 1962
Other groups also demonstrated that the thymus is essential for the generation of immunocompetent lymphocytes and immune responsiveness
Jankovik et al., J Exp Med 116:159-176, 1962; Arnason et al., J Exp Med 116: 177-186, 1962; Waskman et al., J Exp Med 116: 187-205, 1962; Good et al., J Exp Med 116:773-795, 1962
Reif and Allen described the Thy-1 molecule (now CD90) as a cell differentiation marker in thymocytes, T cells and neurones.
Angelo M DiGeorge described the association of thymic aplasia, hypoparathyroidism and infection (DiGeorge´s syndrome). In the same paper Max D Cooper et al., proposed the existence of two types of immune responses: one mediated by lymphocytes generated in the Bursa of Fabricius in birds (B cells) and responsible for the production of antibodies, and the other mediated by lymphocytes differentiated in the thymus (T cells) and responsible for the cell-mediated immune responses
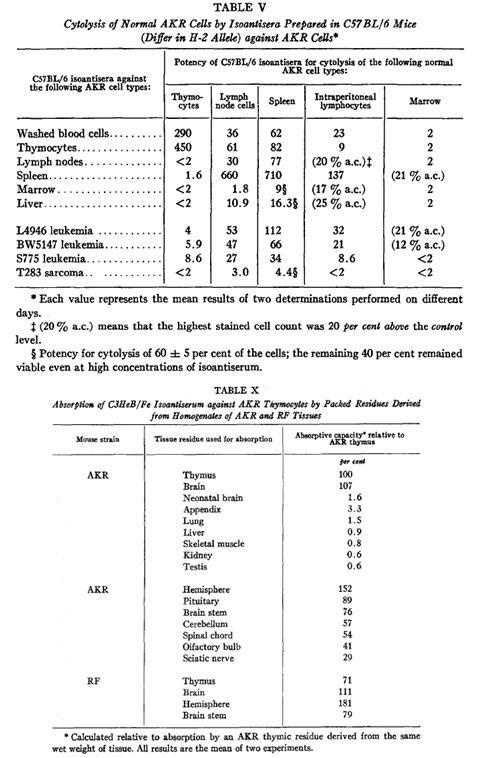
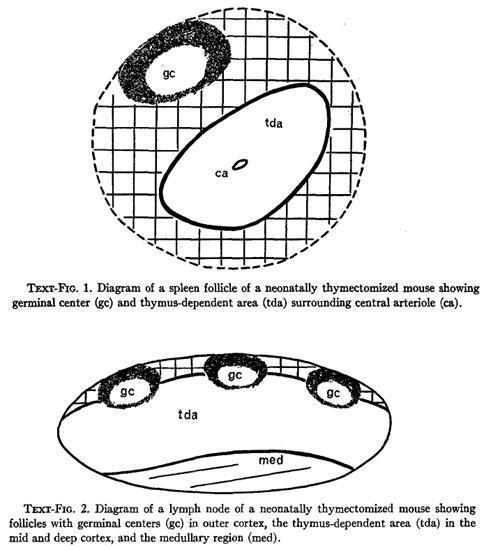
Parrot et al., studied the histological alterations found in the spleen and lymph nodes of neonatally thymectomized mice and proposed that the lymphoid follicles surrounding the central arterioles of the spleen and the mid and deep cortical areas of the lymph nodes are thymic-dependent areas (tda).
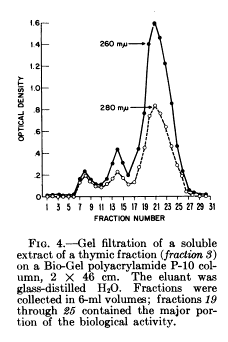
Goldstein et al., isolated from calf thymus a protein with lymphopoietic activity, that they termed Thymosin.
Yunis et al., described the presence of autoimmune events in neonatally thymectomized mice suffering a wasting syndrome, characterized by failure to gain weight, hypothermia, diarrhea and early death.
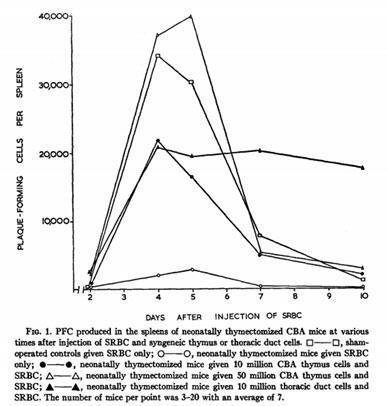
Miller and colleagues in Melbourne demonstrated that antibody-forming cells are bone marrow-derived but require the help of thymus-derived cells to produce antibodies
Mitchell and Miller, PNAS 59: 296-303, 1968; Miller and Mitchell J Exp Med 128: 801-819, 1968; Mitchell and Miller, J Exp Med 128:821-837, 1968
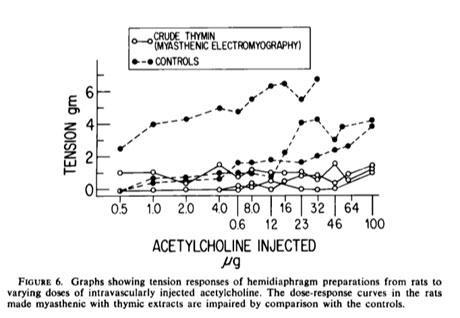
Goldstein and Manganaro reported the isolation from calf thymus of a factor that interferes with neuromuscular transmission and induces the expression of T cell maturation markers. This factor was called Thymin.
Bach and Dardenne isolated the ´Facteur Thymique Sérique´ (FTS) that induces the expression of T cell antigens and restores the mitogen responsiveness in neonatally thymectomized mice.
Coleman et al., described the presence of terminal-deoxynucleotide-tranferase (TdT) in thymocytes.
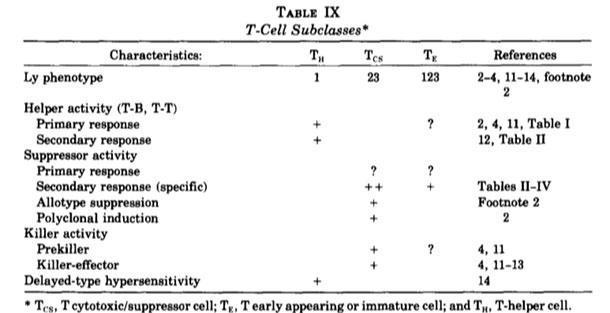
In 1976, Cantor et al., reported that mouse thymocytes can be differentiated into four different subsets according to the expression of the Ly molecules: Ly123- (now CD4-CD8- or double negative, DN), Ly123+ (now CD4+CD8+ or double positive, DP), Ly1+ (now CD4+) and Ly2+3+ (now CD8+).
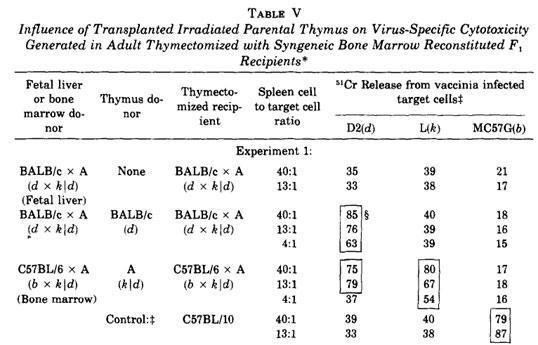
Zinkernagel et al., using thymic chimeras, demonstrated that the thymus is the tissue where the MHC-restricted differentiating T cells are selected.
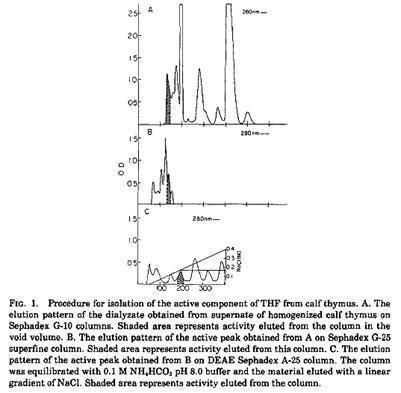
Trainin and colleagues isolated a Thymic Humoral Factor (THF) able to induce immunocompetent cells in spleen from neonatally thymectomized mice.
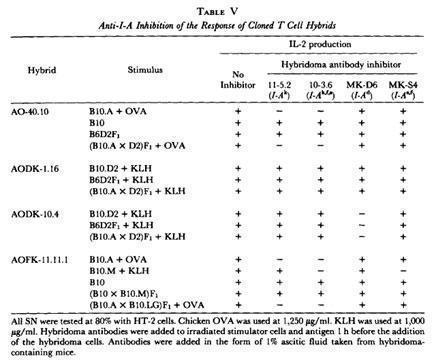
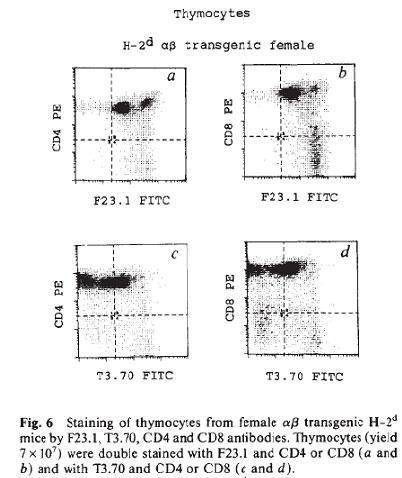
Kappler et al., generated antigen-specific, H-2-restricted, T cell hybridomas that recognize both antigen and H-2 using a single receptor.
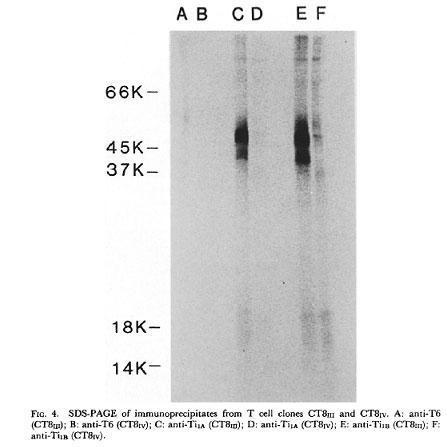
Reinherz and his colleagues (J Exp Med 157:705-719, 1983) using human T cell clones, and Marrack and colleagues (J Exp Med 157: 1149-1169, 1983) using mouse T cell hybridomas obtained monoclonal clonotypic antibodies that identified on the T cell membrane a heterodimer of 90KD associated with the T3 molecule (now CD3).
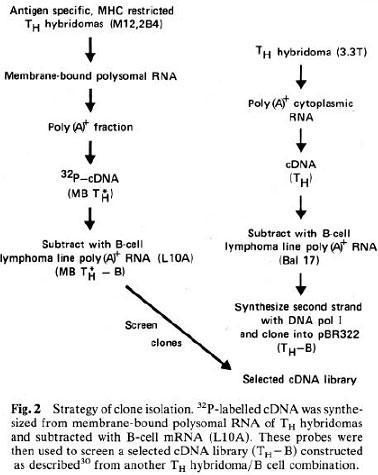
Tak Mak and colleagues using differential screening (Yanagi et al., Nature 308: 145-149) and Mark Davis and colleagues using cDNA substractive hybridization (Hedrick et al., Nature 308: 149-153, 1984; Hedrick et al., Nature 308: 153-158, 1984), independently were able to clone the genes of the T cell Receptor (TCR), elucidate its basic structure, and demonstrate that the TCR genes, like the Ig genes, rearrange at the DNA level to generate the repertoire diversity.
In the late 1980s and early 1990s many groups showed that thymocytes are subject to a positive and negative selection during their intrathymus differentiation and maturation process. Among these groups, von Boehmer and colleagues (Teh et al., Nature 335:229-235, 1988) using TCR transgenic mice demonstrated that thymocytes undergo positive selection in the cortex of the thymus by binding of the TCR with the MHC expressed by the cortical epithelial cells. Also, Marrack and Kappler and their colleagues showed that once thymocytes migrate into the thymus medulla, they undergo a negative selection process leading to apoptosis and tolerance to specific antigens (Kappler et al., Cell 49:273-280, 1987; Kappler et al., Nature 332:35-40, 1988).
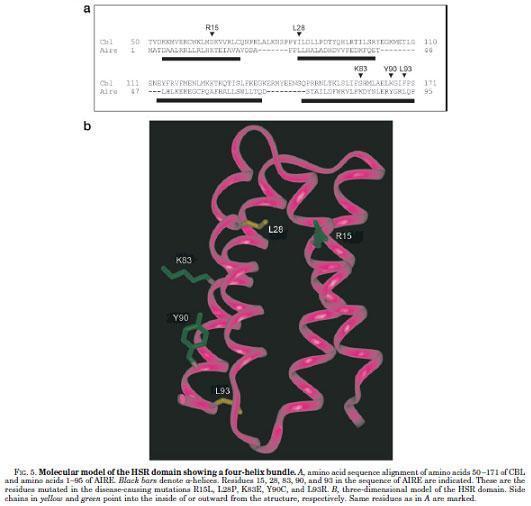
Mutations in the autoimmune regulator (AIRE) gene were identified as responsible for autosomal recessive Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy (APECED) syndrome (Nature Genetics 17:399-403, 1997), also called Autoimmune Polyglandular syndrome type 1 (APS-1), and the human Autoimmune Regulator (AIRE) gene was mapped and cloned (Nagamine et al., Nature Genetics 17:393-398, 1997).
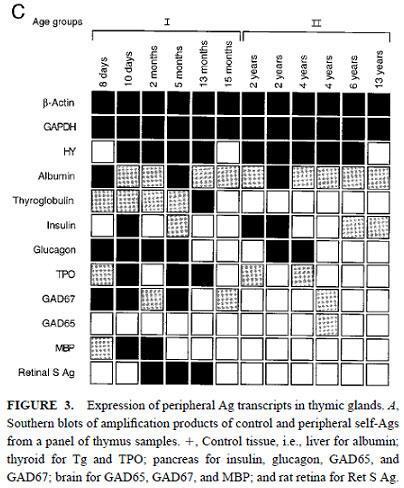
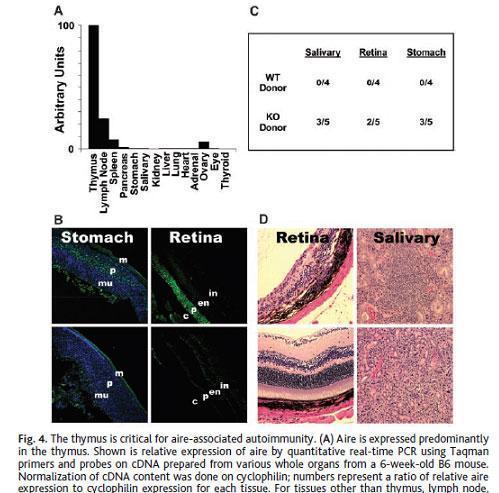
Sospedra et al., demonstrated the presence of a broad range of self-antigens transcripts in human thymus.
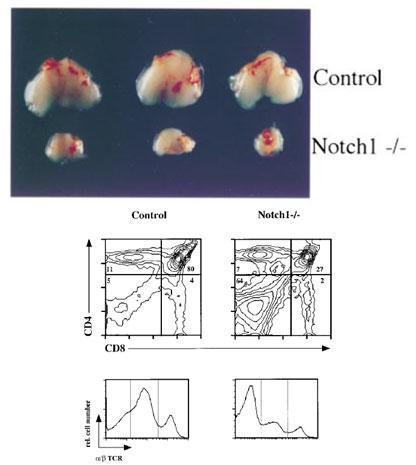
Radtke et al., (Immunity 10: 547-558, 1999) and Pui et al. (Immunity 11:299-308, 1999) demonstrated the crucial role of Notch1 signals in the early T lineage determination.
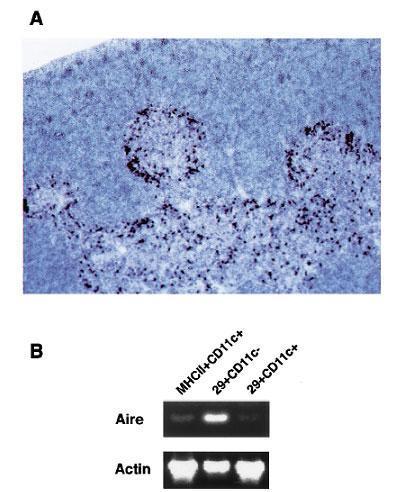
Zuklys et al., demonstrated that AIRE expression is restricted to medullary thymic epithelial cells (mTECs) and thymic dendritic cells and participates in the process of negative selection.
Pitkanen et al., showed that AIRE has transcriptional transactivator properties and interacts with the coactivator CREB-binding protein.
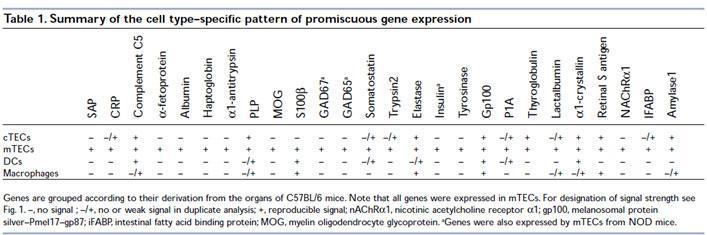
Derbinski et al., demonstrated that mTECs exhibit a promiscuous expression of tissue-restricted antigens (TRAs).
Anderson et al., showed that Aire regulates the expression of a large number of promiscuously expressed genes in mTEC.
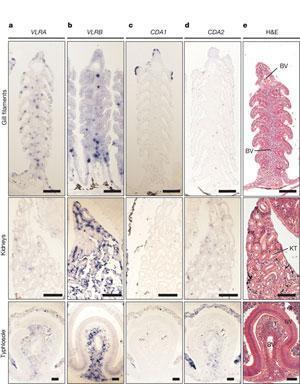
In 2011, Bajoghli et al., found a “thymus-like lympho-epithelial structures, termed thymoids, in the tips of the gill filaments and the neighbouring secondary lamellae (both within the gill basket) of lamprey larvae”
Acknowledgement
History kindly supplied by Dr Luis Garcia – Immunopaedia Steering Committee
Luis F García
Emeritus Professor
Grupo de Inmunología Celular e Inmunogenética
Universidad de Antioquia
Medellín, Colombia
IUIS Education Committee
Immunopaedia Steering Committee