The turn of the 20th century serves as a landmark due to two important events:
- recognition of Gregory Mendel’s work of 1867 and his theory of inheritance, and
- discovery in 1901 of the blood group system on erythrocytes by Karl Landsteiner, an Austrian scientist. This set the pace for search of a similar polymorphic system on leukocytes.
During the first decade of the 20th century, two important advances were made that paved the way to the discovery of the MHC in later years:
- Loeb and Tyzzer discovered that the resistance and susceptibility to transplantable tumors are genetically determined by at least 15 genes.
- Inbred strains of mice were developed by Little (DBA/1, DBA/2, C57BL), Bagg (BALB/c) and Strong (CBA and C3H) that facilitated the understanding of transplant biology.
In 1930, Karl Landsteiner was awarded the Nobel Prize in Medicine and Physiology for the discovery of the human ABO blood system.
Nobel Prize 1930 – The Nobel Prize in Physiology or Medicine 1930 was awarded to Karl Landsteiner “for his discovery of human blood groups.”
Peter A. Gorer in 1936 discovered the antigen II on mouse erythrocytes (Br J Exp Pathol 17:42-50, 1936) and the very next year he reported that this antigen is identical to the gene controlling susceptibility to a transplantable sarcoma (J Pathol Bacteriol 41:691-696, 1937).
In 1944, Peter Medawar (J Anat 78:176, 1944) studying skin transplants in rabbits demonstrated that rejection of homografts (now named allografts) is the result of a specific and systemic immune response.
In 1948, Gorer, Lyman and George Snell published a landmark paper (Proc Royal Soc B 4:9i9, 1948) demonstrating that antigen II was identical to the antigen present on tumors of two strains of mice and was the major factor controlling graft acceptance. The gene coding for this antigen was named H2, where´H´stands for Histocompatibility. The H-2 locus therefore came to be recognized as the Major Histocompatibility Antigen System.
Jean Dausset in 1950s (Vox Sang 4:190, 1954; Acta Haematol 20: 156, 1958; and 20:185, 1958) was the first to describe antibodies to platelets in the sera of multitransfused patients that were able to agglutinate donor leukocytes. He referred to them as leuco-agglutinins.
Jean Dausset (1916-2009) discovered the first HLA antigen in 1958, which he named “MAC”, the three letters based on the first names of three of his volunteers. Later it came to be recognized as HLA-A2.
Rose Payne in the US (J Clin Invest 37:1756, 1958) and Jon van Rood in the Netherlands (Nature 151:1735, 1958) working independently reported the presence of anti-leukocyte antibodies in the sera of pregnant women. This set the momentum for the availability of mono or oligospecific HLA antisera to determine HLA antigens in an individual.
In the 1960s, studies done by Baruj Benacerraf and colleagues (Levine et al. J Exp Med 118:953, 1963) and Hugh McDevitt and coworkers (J Exp Med 126:969, 1967), amongst others, revealed that the MHC genes controlled specific immune responses through what they called Immune Response genes (Ir genes).
In 1960, Peter Medawar received the Nobel Prize for his pioneering studies on tissue transplantation and immunological tolerance. The Prize was shared with McFarlane Burnet who postulated the Clonal Selection Theory of Acquired Immunity.
Nobel Prize 1960 – The Nobel Prize in Physiology or Medicine 1960 “for discovery of acquired immunological tolerance.”
In 1963, Shrefler and Owen (Genetics 48:9, 1053) discovered a mouse serum protein whose levels were genetically determined by genes located on the H-2 loci. They called it ´Ss´, later identified as the C4 component of complement.
In 1964, Bernard Amos organized the First International Histocompatibility Workshop (IHWS) at Duke University, North Carolina, USA, where participants compared various techniques for the detection of human leukocyte antigens. This led to the start of a very productive international collaborative program, which contributed significantly to further define the HLA loci with their multiple alleles. It also discussed the methods to be used for the detection of HLA alleles, and paved the way for studies on the anthropological significance of HLA polymorphism, clinical meaning of HLA and disease associations, and influence of donor-recipient HLA matching and antibody determination in organ and hematopoietic stem cell transplantation. Seventeen such workshops have already been organized, while the 18th IHWS is planned for May 2021 in Amsterdam.
In 1964, Paul Terasaki and John McClelland (Nature 204:998, 1964) introduced the microlymphocytotoxicity test, known as complement dependent cytotoxicity assay or simply CDC, which became the standard serological assay for HLA typing and cross-matching in clinical tissue transplantation.
Also in 1964, Frank Lilly and colleagues (Lancet 2(7371):1207, 1964) reported an association between H-2 and susceptibility to viral leukemogenesis in the mouse system. This was the first publication of a possible association of MHC polymorphisms and disease susceptibility.
In 1967, Fritz Bach and Bernard Amos reported that HL-A genes control the Mixed Lymphocyte Culture (MLC) reaction.
The first report of an association between HLA and a human disease was by Amiel in 1967 (Histocompatibility Testing p79, 1967), who published a weak association of HLA-B5, B15 and BW35 with Hodgkin’s disease.
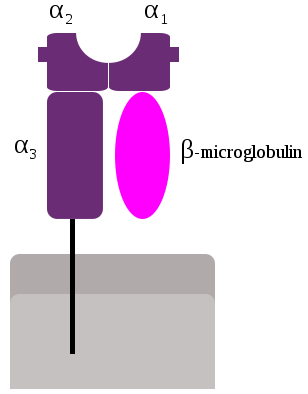
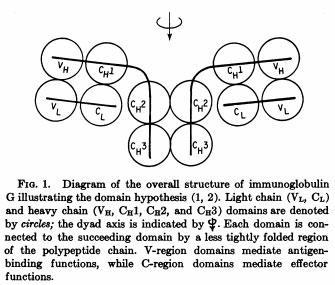
Class I α chain was first isolated in 1968 by Mann et al (Nature 217: 1180, 1968) and Nathenson and Shimada (Transplantation 6:662, 1968).
β2-microglobulin was first isolated by Bergard and Bearn (J Biol Chem 243:4095, 1968) from the urine of patients with cadmium-induced renal tubular damage. The complete protein sequence was published in 1972 by Peterson et al. (Proc Natl Acad Sci USA 69:1697, 1972) indicating that it belonged to the Ig superfamily.
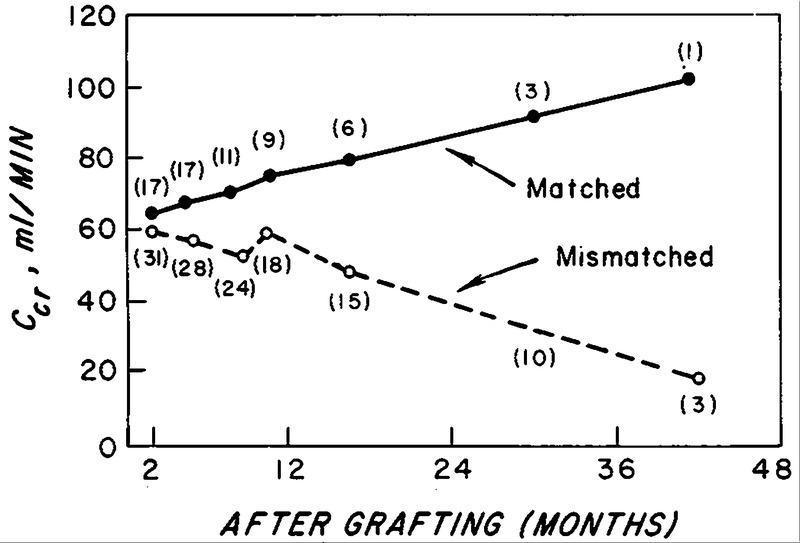
The first evidence that HLA matching improved renal graft survival was published in 1968 (Morris et al., Lancet 2(7572):803, 1968; Patel et al., N Engl J Med 279:501, 1968)
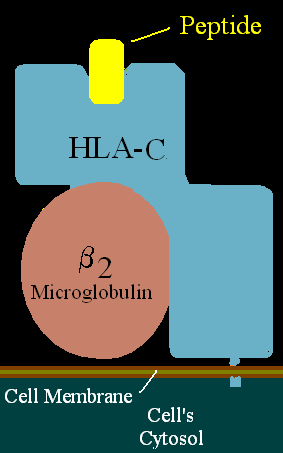
Kissmeyer-Nielsen et al. (Nature 219:1116, 1968) defined the first series of HLA antigens assigned to the HLA-A and B loci.
In 1968, the World Health Organization (WHO) sponsored the establishment of an HLA Nomenclature Committee, which is still active and continues to revise the system.
In 1969, Ceppellini et al. (Transplant Proc 1:385, 1969) provided experimental evidence in man that indicated that the survival of skin grafts depended on HLA compatibility between the donor and the recipient.
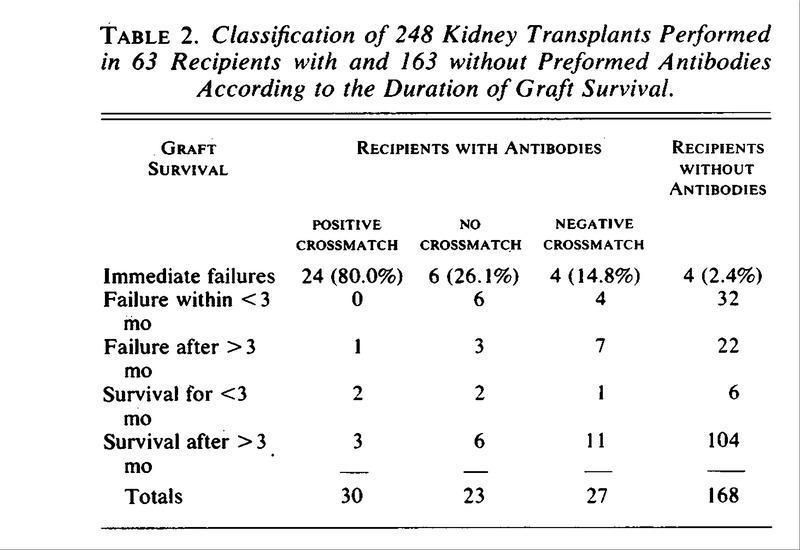
Patel and Terasaki (N Engl J Med 280:735, 1969) demonstrated that the presence of preformed cytotoxic antibodies in recipients of renal grafts was associated with hyperacute rejection.
Sandberg et al. (Histocompatibility Testing p163, 1970) assigned the first serologically defined antigens to the HLA-C locus.
Erik Thorsby (Eur J Immunol 1:57, 1970) and Snell et al. (Transplant Proc 3:183, 1971) independently proposed that the known H-2 genes were encoded by two loci, K and D.
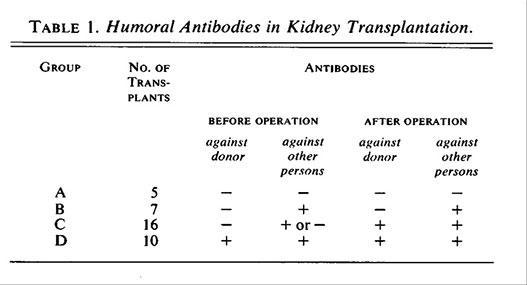
Jeannet et al., (N Engl J Med 282:111, 1970) reported that the development of de novo donor-specific antibodies early after kidney transplantation was associated with severe vascular rejection and poor allograft survival.
Acknowledgement
History kindly supplied by Dr Luis Garcia – Immunopaedia Steering Committee and Narinder K Mehra – All India Institute of Medical Sciences
Luis F García
Emeritus Professor
Grupo de Inmunología Celular e Inmunogenética
Universidad de Antioquia
Medellín, Colombia
IUIS Education Committee
Immunopaedia Steering Committee
Narinder K Mehra
Ex-head, Department of Transplant Immunology and Immunogenetics
All India Institute of Medical Sciences
New Delhi, India