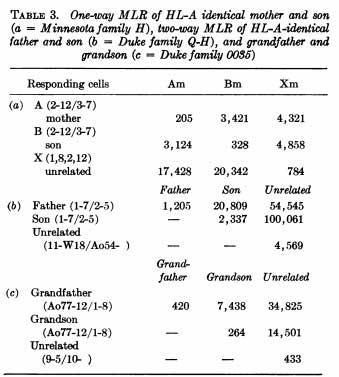
In 1971, Yunis and Amos (Proc Nat Acad Sci USA 68:3031, 1971) showed that the mixed leukocyte reaction (MLR) was controlled by an independent locus (HL-D), centromeric to the serologically detected HL-B.
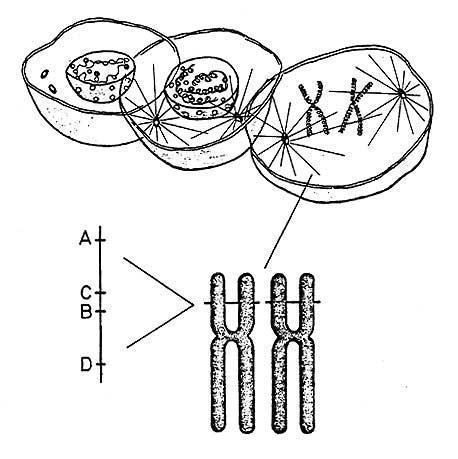
The H-2 complex was assigned to mouse chromosome 17 by Miller et al. (Proc Natl Acad Sci USA 68:1530, 1971)
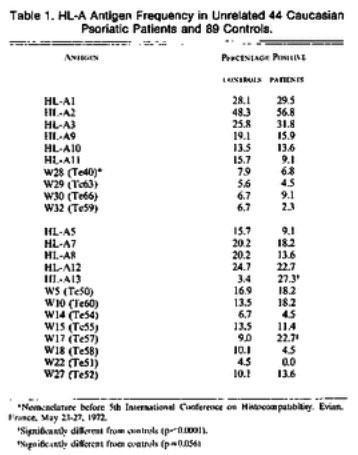
Three groups published their data on the possible association of HLA with human diseases: Falchuck et al (J Clin Invest 51:1602, 1972) reported that the frequency HLA-B8 was increased in patients with celiac disease while Russell et al. (N Engl J Med 287:738, 1972) and White et al (N Engl J Med 287:740. 1972) showed an association of HLA-B13 and B17 with psoriasis.
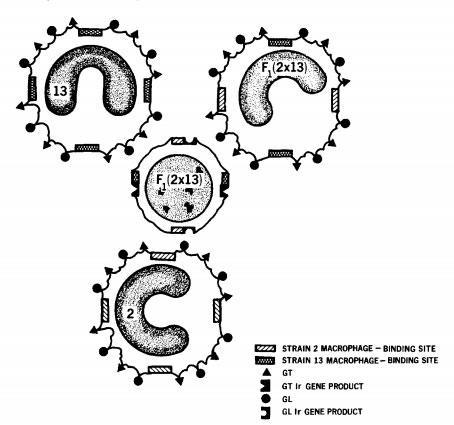
Allan Rosenthal and Ethan Shevach (J Exp Med 136: 1207, 1972; J Exp Med 138: 1194 and 1213, 1973) demonstrated that antigen recognition by guinea pig T cells was regulated by macrophages and restricted by the MHC class II linked Ir genes.
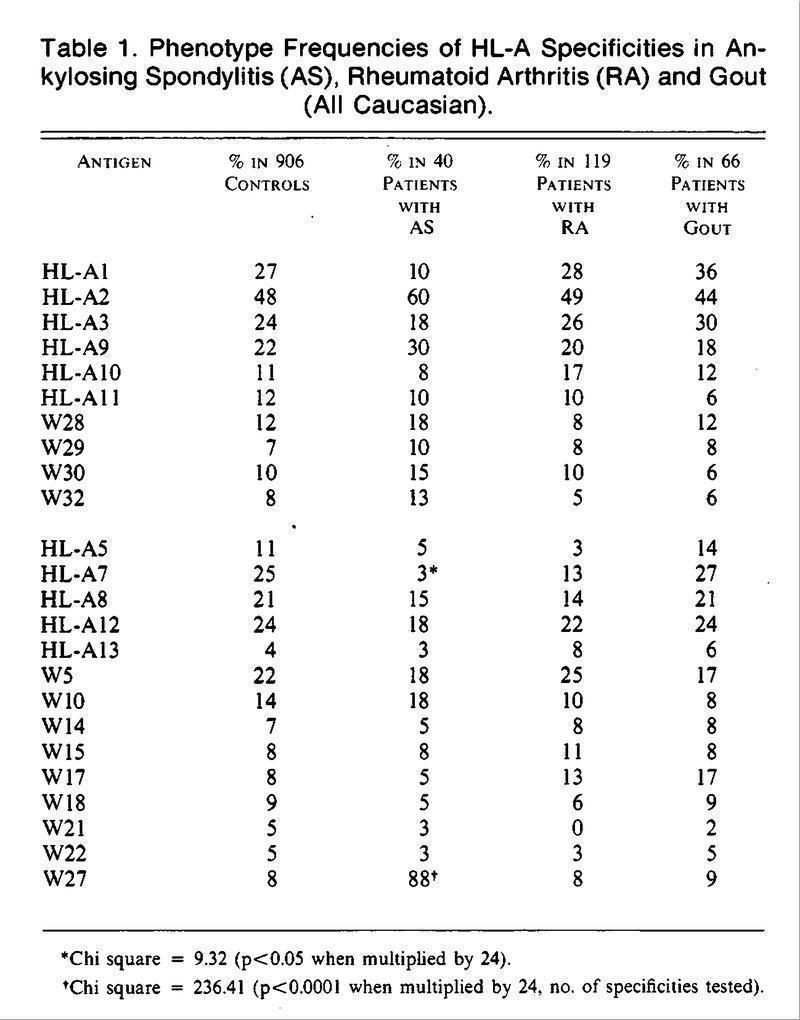
A strong association of HLA-B27 and Ankylosing Spondylitis was reported by Caffrey and James (Nature 242:121, 1973), Brewerton et al. (Lancet 1(7809):904, 1973) and Schlosstein et al (N Engl J Med 288:704, 1973)
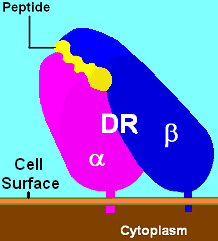
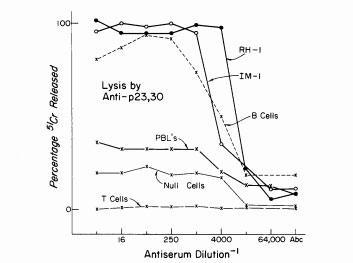
The HLA-DR (HLA-D Related) antigens were serologically detected on B lymphocytes using immunofluorescence (van Leeuwen et al., Transplant Proc 5:1539: 1973)
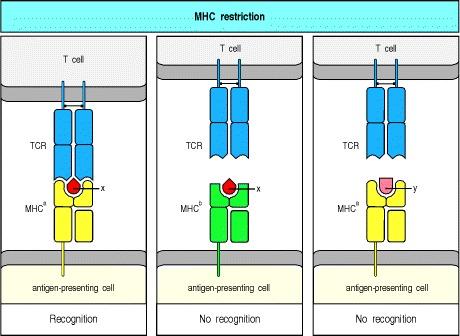
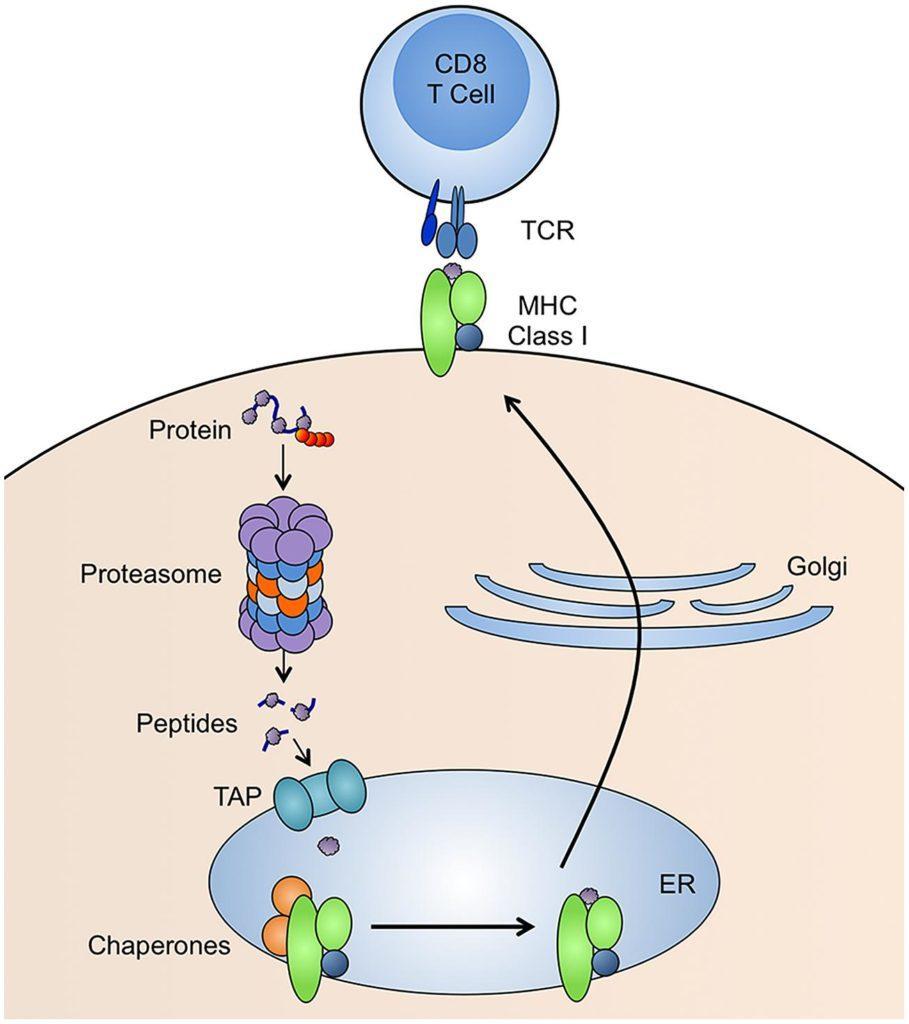
In 1974, Peter Doherty and Rolf Zinkernagel published a series of papers (Nature 248:701, 1974; Nature 251: 547, 1974) demonstrating that recognition of viral infected target cells by CD8+ T cells is restricted by MHC class I genes. They put forward the concept of ‘MHC restriction’.
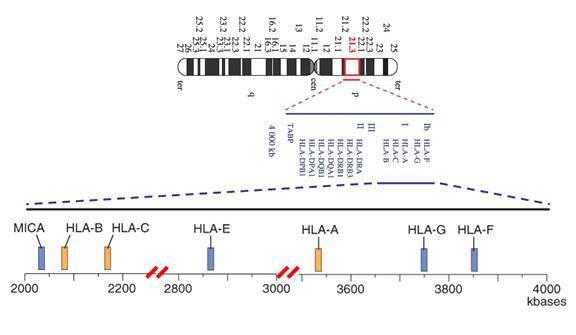
In 1974, van Someren et al. (Proc Natl Acad Sci USA 71:962, 1974) and Lamm et al. (Hum Hered 24:273, 1974) assigned the HLA complex to human chromosome 6.
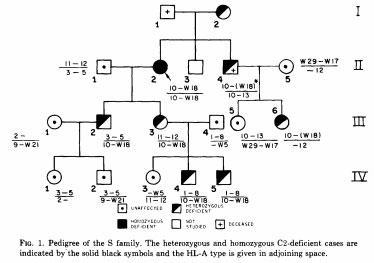
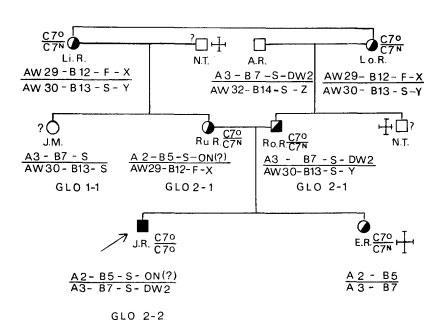
Fu et al. (J Exp Med 140:1108, 1974) demonstrated a linkage between HLA and the gene responsible for complement component C2. Allen (Vox Sang 27:382, 1974) reported that the complement Factor B (Bf) gene is in linkage with HLA genes.
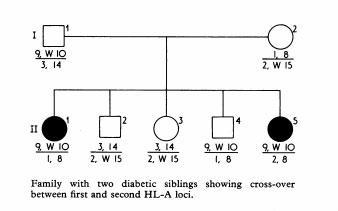
The association of Type I diabetes (IDDM) and HLA was first reported by Singal et al. (Diabetes 22:429, 1973) and confirmed by the classical studies of Nerup and colleagues (Lancet 2(7885): 864, 1974) and Cudworth and Woodrow (Br Med J 3:133, 1975).
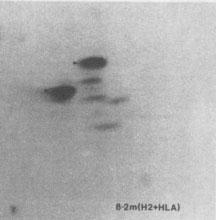
Goodfellow et al. (Nature 254:267, 1975) and Smith et al. (Am J Hum Genet 39:21, 1975) assigned the human b2-microglobulin locus to chromosome 15.
Rittner and colleagues (Histocompatibility Testing, p945, 1975) demonstrated a linkage between HLA genes and the complement component C4.
In 1977, Els Goulmy et al. (Nature 266:544, 1977) demonstrated that antigen recognition by CD8 T cells was restricted by class I molecules, while Bergholtz and Thorsby (Scand J Immunol 6:779, 1977) showed that class II molecules restrict antigen recognition by CD4 T cells.
The class II α and β chains were identified during the late 1970s by many groups, for both H-2 (Cullen et al. Transplantation 30:236, 1976; Freed et al. J Immunol 121:91, 1978; Cook et al. J Immunol 123:2799, 1979) and HLA-DR systems (Humpreys et al. J Exp Med 144:98, 1976; Kaufman and Strominger, Proc Natl Acad Sci USA 576:6304, 1979).
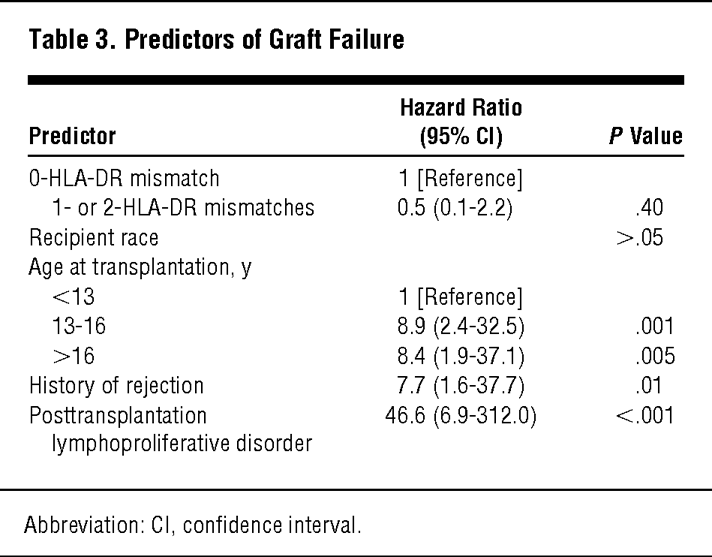
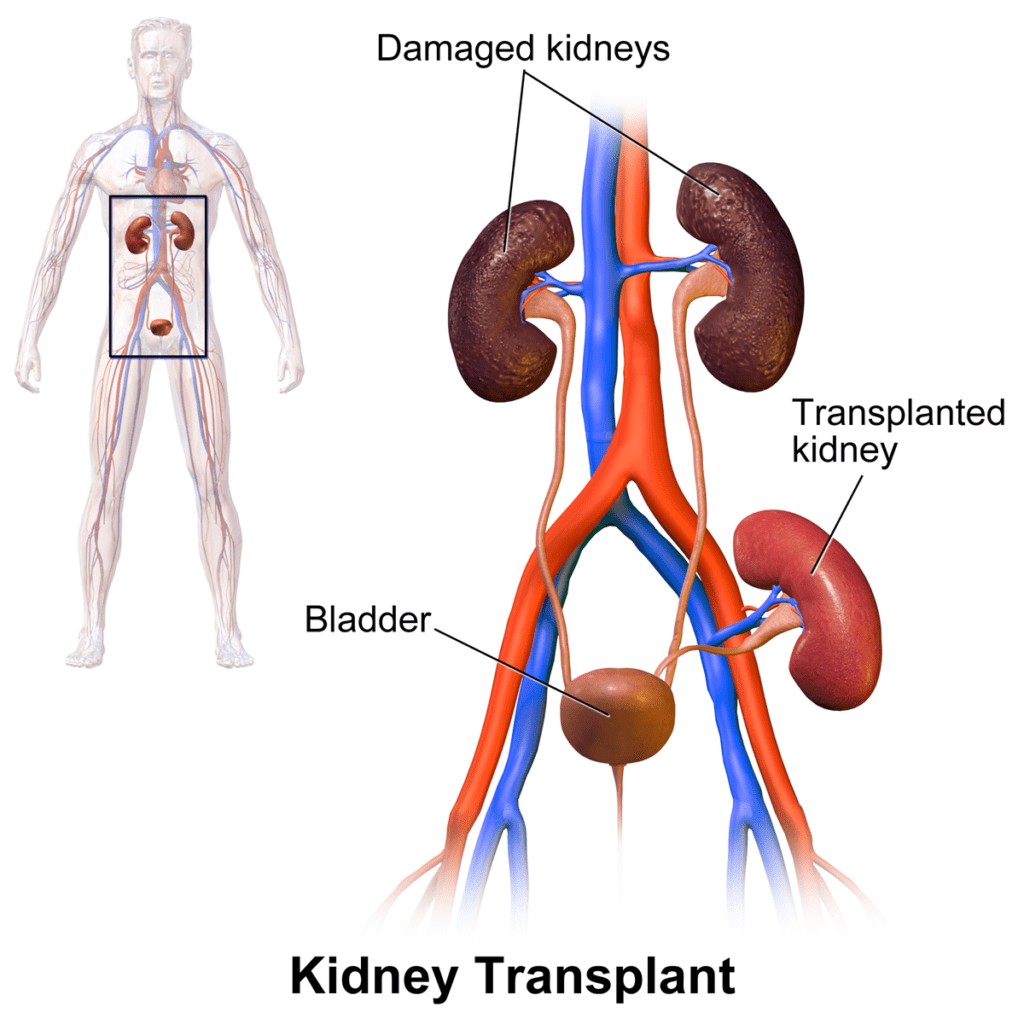
Ting and Morris (Lancet 1(8064):575, 1978) and Albrechtsen et al. (Lancet 2(8100):1226, 1978) reported a strong effect of HLA-DR matching on kidney graft survival.
Jones et al., (Mol Immunol 16:51, 1979) reported the existence of an invariant chain (Ii) that binds the α and β class II chains in the endoplasmic reticulum (ER), before the trimer moves to the Golgi apparatus.
In 1980, Jean Dausset, George Snell and Baruj Benacerraf were awarded the Nobel Prize in Medicine and Biology for their discoveries on the MHC. Peter Gorer could not make the list because he had passed away in 1961.
Nobel Prize 1980 – Discoveries concerning genetically determined structures on the cell surface that regulate immunological reactions.
In 1982, Gerhard Opelz, at the University of Heidelberg (Germany), launched the Collaborative Transplant Study (CTS), an international collaborative program involving a large number of transplant groups and tissue typing laboratories. The data collected through CTS clearly demonstrated an important effect of HLA matching on kidney graft survival. The CTS has been a great source of very important information on factors influencing long-term graft survival following organ transplantation.
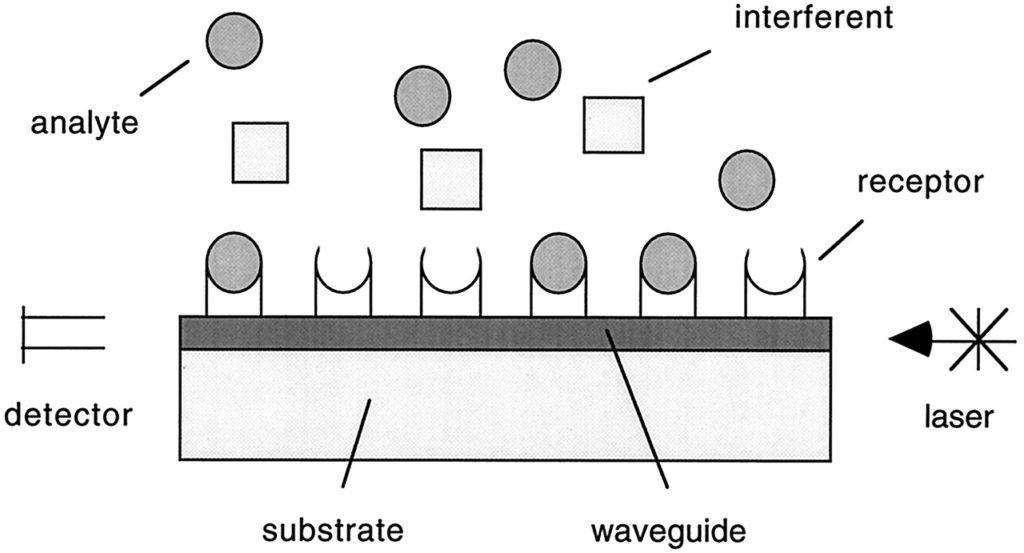
Garovoy et al. (Transplant Proc: 15:1939, 1983) introduced flow cytometry as the more sensitive technique for the pre-transplant crossmatch analysis.
Bernabeu et al. (J Immunol 131:2032, 1983) reported the production of single recombinant HLA molecules, opening the possibility to use these molecules for the detection of antigen-specific responses.
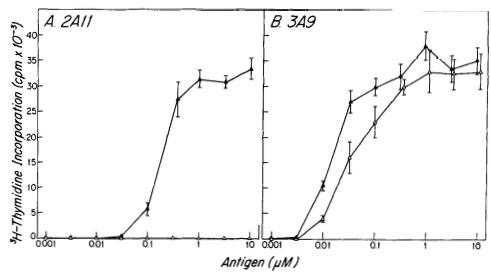
In 1985, Emil Unanue and his colleagues described the characteristics of the peptides binding H-2k (Class I) (J Immunol 135:368, 1985) and I-Ak (Class II) (Nature 317:359, 1985) molecules.
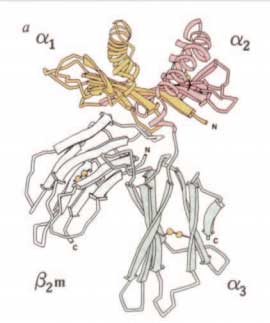
In 1987, Pamela Bjorkman, Jack Strominger and colleagues reported the three-dimensional structure of HLA-A2 by X-ray cristallography (Nature 329: 506, 1987; and 329:512, 1987). They demonstrated that polymorphic amino acid residues located in the floor or side chains of the peptide binding groove of HLA class I molecules bind antigenic peptides in specific residues, while other residues bind the T-cell receptor (TCR).
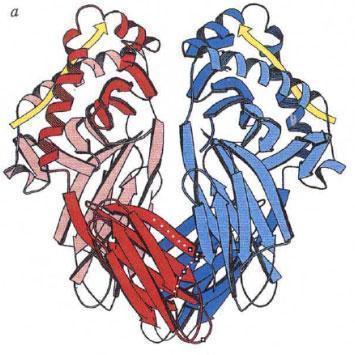
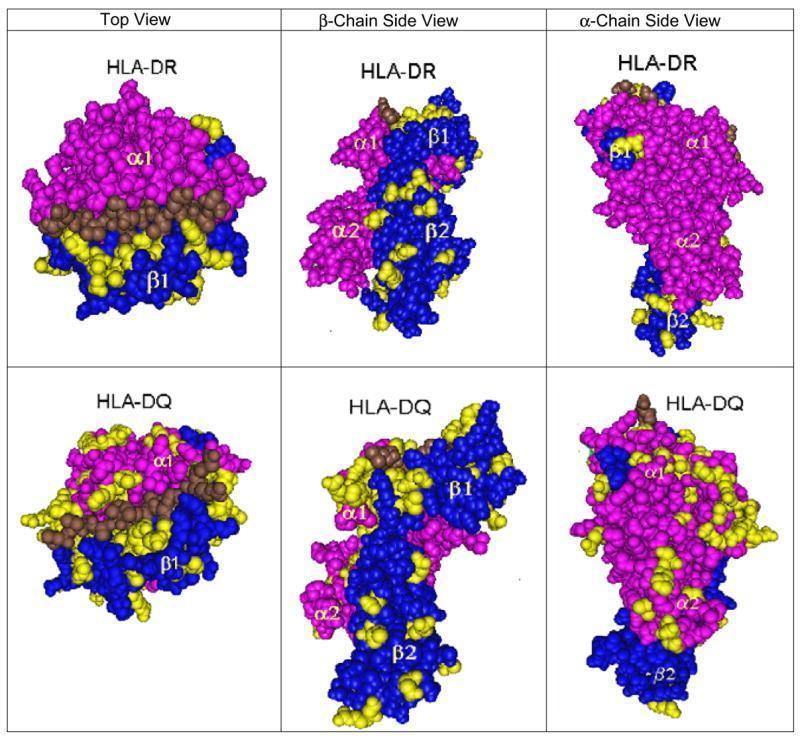
The three-dimensional structure of the HLA Class II molecules was proposed by Brown et al. in 1988 (Nature 332:845, 1988) and confirmed by the same group for HLA-DR1 (Nature 364:33, 1993).
Roche and Creswell (Nature 345:615, 1990) demonstrated that a part of the invariant chain Ii, the class II-associated Ii peptide (CLIP), temporarily blocks the peptide-binding groove of nascent class II molecules.
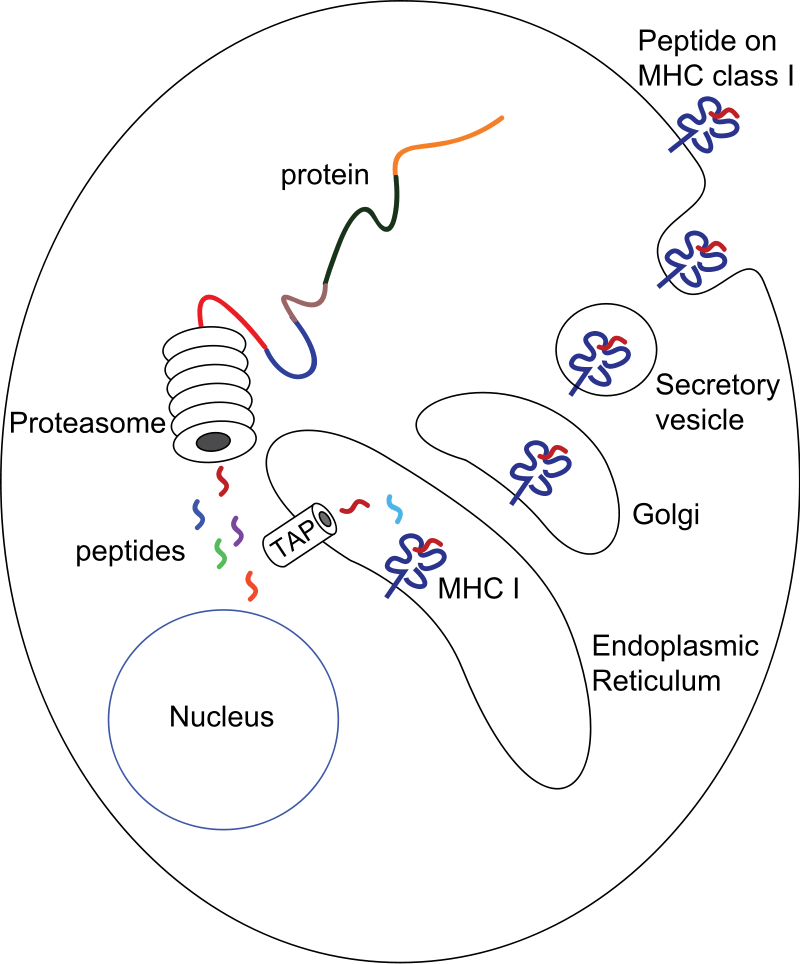
In 1990, three groups simultaneously demonstrated that a gene located on the class II region regulates the class I antigen presentation (Deverson et al., Nature 348:738, 1990; Trowsdale et al., Nature 348:741, 1990; Spies et al., Nature 348:744, 1990). The gene codes for an ATP-binding cassette (ABC) superfamily of transporter proteins that transport peptides from the cytoplasm into the ER, where peptides are loaded into nascent class I molecules. The protein was named TAP.
In 1990, Halloran and colleagues (Transplantation 49:85:1990) demonstrated that de novo produced, donor-specific HLA class I antibodies can induce acute rejection of kidney grafts.
In 1991, a proteasome gene was found in the HLA class II region (Glynne et al, Nature 353:357, 1991). Soon after, Rock et al (Cell 78:761, 1994) demonstrated that the low molecular weight proteasome (LMP) was involved in the generation of peptides that are then transported into the ER by TAP.
In 1991 Rotzschke et al (J Exp Med 174:1059, 1991) demonstrated that cytotoxic T lymphocytes could directly recognize allogeneic MHC-peptide complexes, without presentation by autologous antigen presenting cells. This phenomenon is known as direct allorecognition.
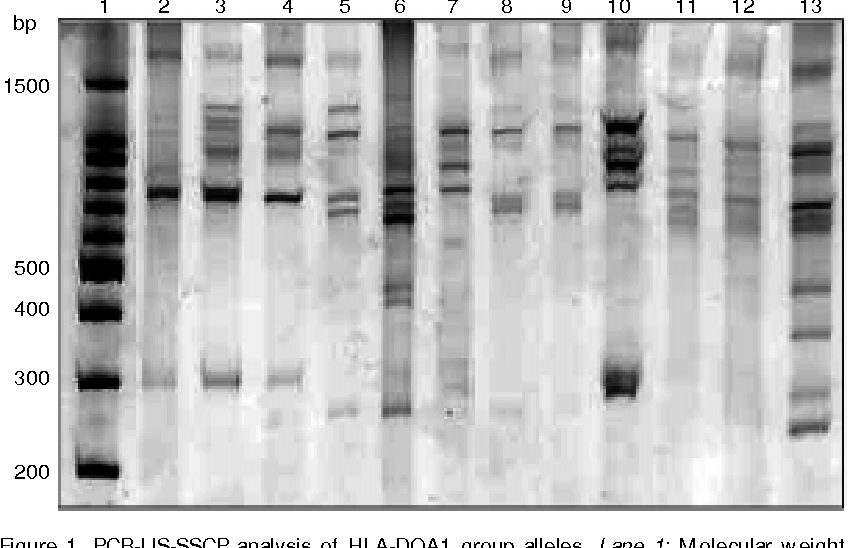
Following the development of the Polymerase Chain Reaction (PCR) by Saiki (Science 230:1350, 1985), several DNA based procedures were developed for molecular HLA typing. This includes: Sequence Specific Oligo hybridization (SSOP) (Wordsworth, Immunol Lett 29:37, 1991) , PCR based Sequence Specific Primer technology or PCR-SSP (Olerup and Zetterquist, Tissue Antigens 39:225, 1992), PCR-Single Strand Conformation Polymorphisms or SSCP (Carrington et al., Hum Immunol 33:208, 1992), and Sequence Based Typing or SBT (Cereb et al., Tissue Antigens 45:1, 1995). These molecular methods have since replaced the serological methods for HLA typing in most clinical laboratories.
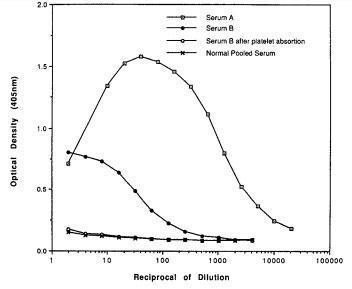
In 1993, Kao et al (Transplantation 55:192, 1993) introduced ELISA as an immunoassay for the detection of anti-HLA antibodies.
In 1993, Steimie et al (Cell 75:135, 1993) demonstrated that the ‘Bare Lymphocyte Syndrome’, characterized by the complete lack of HLA class II expression leading to severe combined immunodeficiency, is caused by deletion in a gene that regulates the expression of class II molecules, the Class II Transactivator (CIITA).
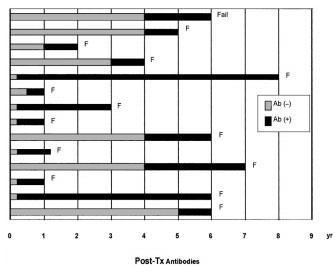
In 1995 Buchler et al (Transplant Proc 27:2478, 1995) reported that the development of post-transplant anti-HLA antibodies is associated with chronic rejection.
In 1996, Peter Doherty and Rolf Zinkernagel shared the Nobel Prize for Medicine and Biology for their discovery on the role of MHC class I antigens in the recognition of viral-infected target cells by CD8+ T cells and “MHC restriction”.
Nobel Prize 1996 – discoveries concerning the specificity of the cell mediated immune defence
In 1997, Fulton et al (Clin Chem 43:1749, 1997) introduced the multiplex analysis technique for detection of HLA antibodies.
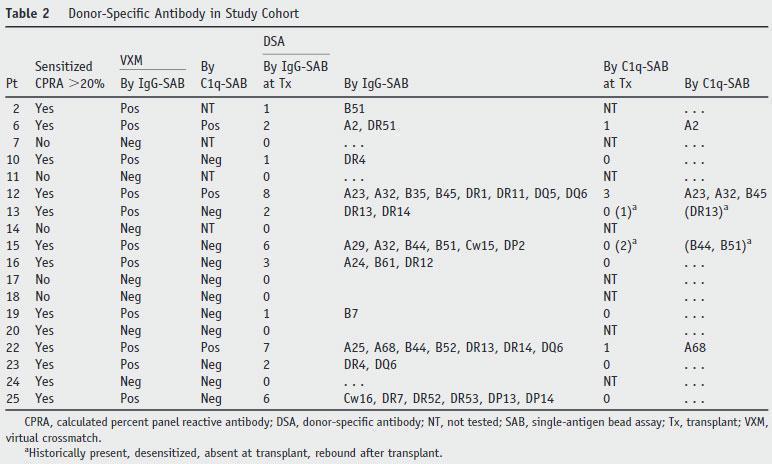
In 2002, Rene Duquesnoy (Human Immunol 63:339, 2002) introduced the HLA Matchmaker algorithm that allows to calculate the level of HLA sensitization.
In 2002, Terasaki´s group reported that all chronic rejection kidney failures were preceded by the development of HLA antibodies (Transpl 74:1192, 2002)
Since 2008, role of MICA (MHC class I associated) allelic polymorphism and the possible influence of non-HLA antibodies in organ transplantation is a subject of intense debate.
In 2011, Chin et al (J Heart Lung Transplant 30:158, 2011) introduced the C1q binding assay for detection of Complement-activating HLA antibodies.
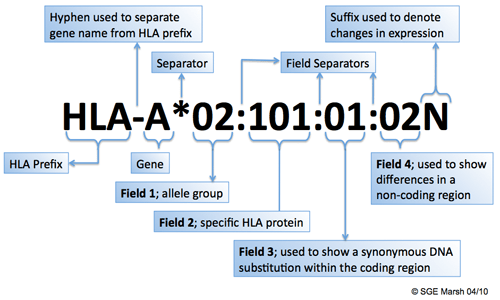
In 2011 the WHO Nomenclature Committee for Factors of the HLA System updated the rules for the nomenclature of HLA loci and alleles (Marsh , Tissue Antigens 78:482, 2011; http://hla.alleles.org/nomenclature/naming). Presently, >18000 HLA alleles (HLA class I 13,324 and HLA class II 4857 have become known primarily due to extensive improvements in molecular based technologies.
The next two decades will see developments in regenerative medicine/biology to re-create and/or repair damaged organs and a switch from standard to patient specific treatment.
Immunogenetics of the host, beyond MHC will make us understand why some organs survive despite having so many so many incompatibilities, while others are lost even though they are optimally matched. Prediction will become the key word for transplantation.
Acknowledgement
History kindly supplied by Dr Luis Garcia – Immunopaedia Steering Committee and Narinder K Mehra – All India Institute of Medical Sciences
Luis F García
Emeritus Professor
Grupo de Inmunología Celular e Inmunogenética
Universidad de Antioquia
Medellín, Colombia
IUIS Education Committee
Immunopaedia Steering Committee
Narinder K Mehra
Ex-head, Department of Transplant Immunology and Immunogenetics
All India Institute of Medical Sciences
New Delhi, India