Introduction
- Burnet and Thomas originally suggested that there is continual immune surveillance and elimination of malignant cells which spontaneously arise due to mutations
- The surveillance concept led to a more complete concept of immune editing, in which the immune response eliminates most potentially malignant cells, but later struggles to maintain equilibrium and tumour clones prone to escape are selection
- Although immunodeficiency predisposes to cancer, most human cancers develop in immunologically intact hosts
- Progression of tumours from low-grade, localised disease to widespread metastases involves interaction between tumour cells and host immunity
- A tumour cell arises and evolves, under pressure from immune system, from a premalignant to a malignant and a metastatic state if it is not eradicated
- Immune molecules (e.g. TGF-β) can have beneficial effects in the premalignant state, but detrimental effects in later stages, promoting tumour progression (Figure 1)
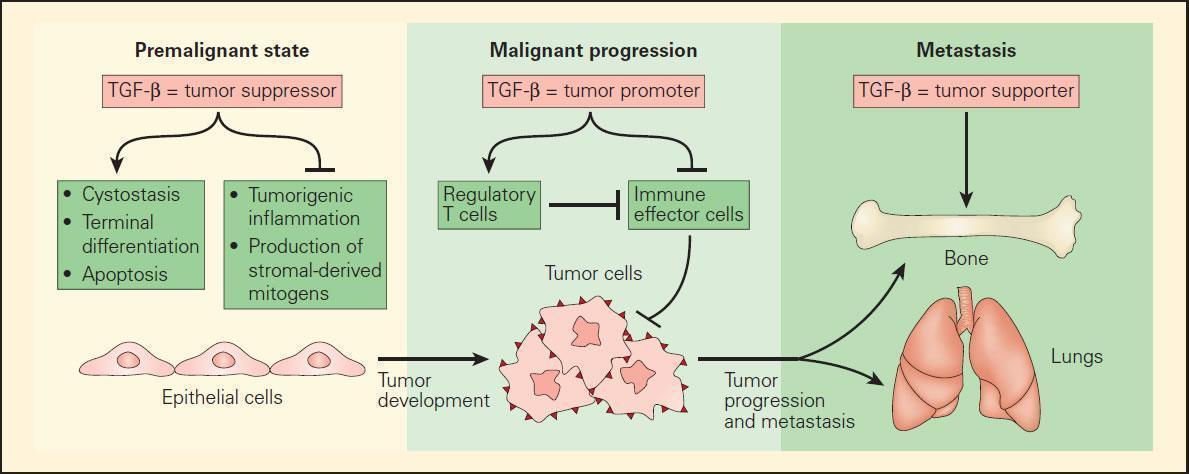
Figure 1. Schematic representation of the role of TGF-b in mediating the tumour development in an epithelial cell target progressing from the premalignant to the malignant state and terminating in the metastatic state. In the premalignant state, TGF-β functions as a tumour suppressor by blocking the expression of stromal-derived mitogens and suppressing protumourigenic inflammation. In this initial state, TGF-β supports the cytostasis, terminal differentiation, and apoptosis of premalignant cells, which have either an overexpressed oncogene or suppressed tumour-suppressor gene. Once the epithelial cells become fully malignant, TGF-β has the opposite effect by blocking the antitumour immune response either directly or through Treg cells. Once a tumour is established, TGF-β further supports the formation of metastases to several distal sites, including bone and lung tissues. [Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGF beta. Nat Rev Immunol. 2010;10:554–67.]
- Anti-tumour immune responses include the complete cell array of innate and adaptive system effectors
- The immune response to cancer is thwarted not only by low immunogenicity of cancer cells, but by its ability to divert an effective effector T-cell cytolytic response towards an ineffective tolerant or anergic state, mediated primarily by Treg and associated immunosuppressive cytokines, especially TGF-β
- Succesful treatment of human tumours requires understanding of the effector mechanisms participating in control of tumour progression
- Tumour progression from low-grade, localised disease to widespread metastasis clearly involves the interaction between tumour cells and the host immune system.
- One result of the “war on cancer” that was launched in the mid-1970s has been the veritable explosion in our understanding of the biology of cancers and immune response to malignancies, that led to the development of new chemotherapies, therapies targeting specific pathways and immunotherapies.
- Most studies of cancer immunotherapy have focused on augmenting tumour immunity through supplementing active immune elements such as the use of monoclonal antibodies (Table 1), vaccines directed against tumour-associated antigens (tumour peptides), adoptive cell therapy (e.g. T effector cells transfusion) and the use of cytokines (IL-7, IL-15, and IL-21), or inhibitors of cytokines (TGF-β) or their signaling pathways (CTLA-4).
Clinical Applications
- The goal of immunotherapy is to activate tumouricidal pathways to lyse cancer cells in a targeted manner.
- Various new chemotherapies and immunomodulatory therapies target specific pathways (e.g. the epidermal growth factor pathway, which may drive tumour progression).
- Newer immunotherapies have demonstrated significant anti-cancer activity, including shrinkage of tumours in lung cancer, lymphoma, leukemia, renal cell carcinoma, with the most notable effect in colorectal cancer and malignant melanoma.
- A few immunotherapeutic strategies are discussed:
- Use of monoclonal antibodies
- Vaccines directed against tumour-associated antigens (tumour peptides)
- Adoptive T cell transfusion (T effector cells)
- Use of cytokines (IL-7, IL-15, and IL-21) or inhibitors of cytokines (TGF-β) or their signaling pathways (CTLA-4)
Monoclonal Antibodies
- One of the most significant achievements in immunology in recent years has been the development and availability of powerful, highly specific monoclonal antibody (mAbs) preparations for treatment of a variety of clinical conditions, including human malignancy (Table 1).
- Most recently with the increasing recognition of the importance of Treg cells, Th17 cells, and the pivotal role of TGF-β in directing the balance and plasticity of these CD4+ T cell subsets, a major investigative effort has been directed to the development of products that may inhibit TGF-β signaling pathways.
- Highly-specific monoclonal antibodies (Table 1) are used to treat various clinical conditions, including malignancy.
- Antibody-mediated immunotherapy (passive IV administration) has significant effects on various tumours.
- Although the precise effector mechanisms of these antibody agents is unclear, their postulated mechanisms of action are varied and include perturbation of growth factor-related signaling, antibody-complement mediated toxicity and ADCC, and inhibitory effects on growth factors that result in blockage of angiogenesis.
- Other advances of monoclonal antibody therapy include conjugation of radioactive or other cytotoxic ligands to the antibody thus allowing the antibody to serve as a carrier molecule for other cytotoxic molecules, e.g., iodine-131 tositumomab and lutetium-177 (177Lu) for the treatment of CD20+ follicular non-Hodgkin’s lymphoma and prostatic cancer, respectively.
| Product | Target | Postulated mechanism of action | Clinical use |
|---|---|---|---|
| Cetuximab | Human epidermal growth factor receptor (EGFR) | Growth inhibition of EGF by binding to EGFR | EGFR-expressing, metastatic colorectal carcinoma, head and neck cancer |
| Panitumumab | |||
| Matuzumab | |||
| Trastuzumab | Growth factor receptor protein (HER2) | Neutralization by binding with HER2 | Breast cancer |
| Rituximab | CD20 antigen on the surface of B cells | Complement-dependent and ADCC mediated cytotoxicity | Diffuse large B cell lymphoma (DLBCL), CD20+, non-Hodgkin's lymphoma, rheumatoid arthritis |
| Iodine-131 tositumomab and yttrium-90 ibritumomabtiuxetan | CD20 antigen on the surface of B cells | Same as rituximab, and ionizing radiation from the radioisotope | B cell non-Hodgkin's lymphoma (NHL) |
| Bevacizumab | Human vascular endothelial growth factor (VEGF) | Antiangiogenesis effects by binding VEGF and preventing its interaction with its receptors | Metastatic carcinoma of the colon or rectum, lung cancer, breast cancer, renal cell carcinoma |
Table 1: Clinical Use of Monoclonal Antibodies for Cancer Immunotherapy. [Bellanti, JA (Ed). Immunology IV: Clinical Applications in Health and Disease. I Care Press, Bethesda, MD, 2012]
Augmenting Anti-tumour Cytotoxic Responses
- Strategies to augment the generation of antitumour cytotoxic responses include the adoptive transfer of in vitro expanded patients’ blood leukocytes or tumour-infiltrating lymphocytes (TILs), vaccines to immunize against TAAs and gene therapy, among others.
- Successful proof of principle studies show their immunotherapeutic effects in cancer (i.e. generation of anti-tumour CTL in vivo).
- Clinical responses are however suboptimal and require further research and modification, including identification of the optimal combinations of chemotherapy and immunotherapy.
- Even with adequate anti-tumour cytotoxic responses, tumour cells may still develop resistance mechanisms, in which case sensitizing agents may be required to overcome this resistance.
- A better understanding of the immune system, tumour microenvironment, and bi-directional communication between these and tumour cells will facilitate development of better therapeutic strategies.
Cytokines & Chemokines
- Because leukocyte-extracted interferon produced occasional beneficial responses in renal cell carcinoma and malignant melanoma patients, recombinant IFN-α therapy was developed.
- The mechanism of action is thought to be generalised enhancement of innate and adaptive immune responses.
- Clinical trials demonstrate IFN-α efficacy (disease resolution) in <10% of patients, but responses may be durable (up to decades).
- Potential toxicity is considerable, most commonly flu-like symptoms, depression, cytopaenias (leukopenia, anemia), abnormal liver function, weight loss and the development of autoimmune disorders.
- These limit the use of interferon, but other cytokines and/or chemokines (IL-7, IL-15, and IL-21, CXCL12) have shown promise and they are largely investigational at present.
Adoptive Cell Therapy
- Adoptive cellular therapy (ACT) is defined as the infusion of immune effector cells for the treatment and/or prevention of disease.
- The idea that lymphocytes extracted from a tumour environment may be sensitised and therefore may have the potential to induce tumour regression has led to the use of TILs in clinical trials.
- Adoptive cell therapy requires generation of highly avid tumour-reactive T-cells derived from tumour-infiltrating lymphocytes (which can, for example, be efficiently isolated ex vivo from melanoma lesions using high levels of IL-2)
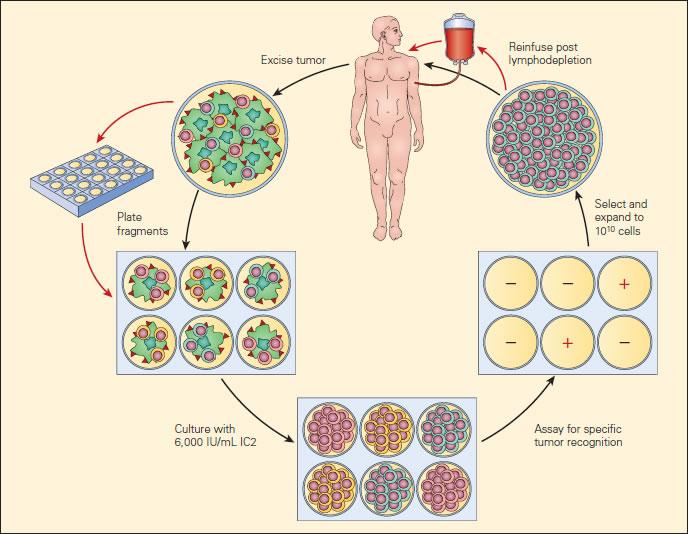
Figure 2. Current clinical protocols for adoptive cell therapy (ACT). [Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308.]
- More recently, an exciting development has been a modification of adoptive cellular therapy by the use of gene-modified lymphocytes. Genes encoding TCRs isolated from high avidity T cells that recognise cancer antigens have been inserted into circulating human lymphocytes using retroviral or lentiviral vectors to redirect lymphocyte specificity to these cancer antigens.
- Tumour specific T cells, derived from TILs, can be efficiently isolated ex vivo from melanoma lesions using high levels of interleukin-2 (IL-2). TILs are successively selected for their ability to secrete high levels of interferon-γ (IFN-γ) when cultured with autologous or allogeneic MHC-matched tumour-cell lines.
- Alternatively, cell-mediated lysis has been used to identify tumour-reactive T cells for transfer.
- Highly avid, tumour-antigen-reactive T cell populations selected for ACT are rapidly expanded (to up to 1010 cells) using CD3-specific antibody, exogenously supplied IL-2, and irradiated allogeneic peripheral-blood mononuclear “feeder” cells. Cells are validated for activity before transfer.
- Patients now receive systemic immunosuppression (cyclophosphamide, fludarabine or total body irradiation) before the adoptive transfer of antitumour lymphocytes.
- In the largest clinical TIL trial to date, 60 percent of metastatic melanoma patients who had not previously received any immunotherapy showed objective responses, although the characteristics of the tumour environment that produces TILs in vivo remain unclear.
Anti-tumour Vaccines
- Various vaccine approaches have been evaluated, ranging from preparation of whole autologous tumour cells ± adjuvants, to genetically prepared products engineered to cross-react with tumour antigens (e.g. peptides targeting CEA and PSA).
- Both approaches show evidence of tumour regression, but responses in the setting of advanced disease are rare.
- Some postulate that vaccines will achieve their greatest utility in a preventative or adjuvant setting.
- Unfortunately, several phase II trials of cancer vaccines for advanced malignancies show inferior outcomes compared to control groups (immunisation may select for development of more aggressive tumour clones).
Targeting Treg
- After diagnosis, cancer patients are subjected to traditional anti-tumour therapy (including surgical debulking, radiation, chemotherapy, and anti-angiogenic therapy).
- Depending on the clinical situation, patients receive a combination of these.
- Traditional therapy often cannot target the tumour itself, focusing either on anatomic location or on rapidly-dividing cells.
- Most studies of cancer immunotherapy have focused on augmenting tumour immunity via enhancing anti-tumour immunity.
- However, reversing tumour-related immunosuppression is also of interest, and newer studies targeting suppressive molecules and Treg may yield successful tumour immunotherapy. (Figure 3)
- Poor performance in clinical trials thus far probably reflects inadequate knowledge of the immune response to cancer and optimal therapy combination schedules.
- Two additional approaches demonstrate promise in gastro-intestinal cancer: use of APC and autologous tumours, and use of viral therapy (e.g. vaccinia vectors bearing tumour antigens and co-stimulatory molecules).
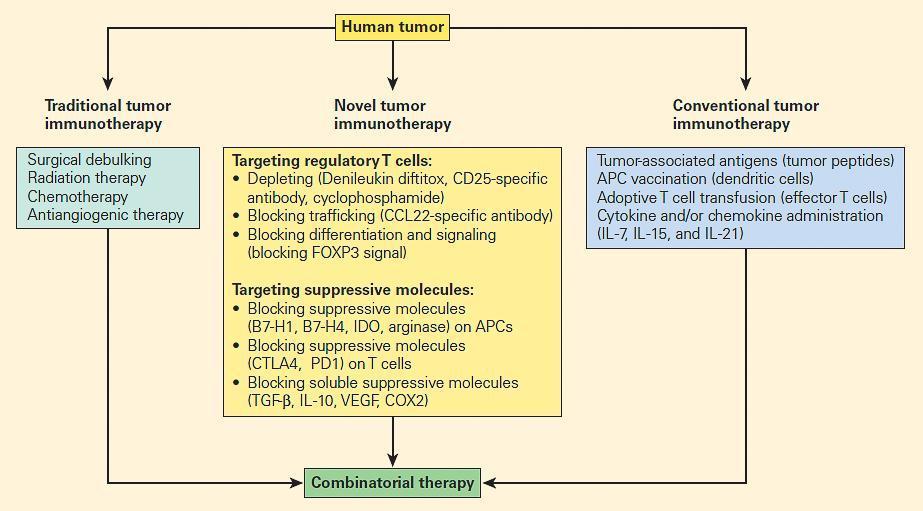
Figure 3. Comparison of traditional tumour therapy, conventional immunotherapy, and novel forms of immunotherapy, including therapeutic targeting of suppressive mechanisms, including regulatory T cells. [Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307.]
Links
- Galluzzi, L. et al. Classification of current anticancer immunotherapies. Oncotarget. (5)24. (2014)
- Kroemer, G. et al. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. (31):51–72. (2013)
- Mickaël, M. et al. Autophagy-Dependent Anticancer Immune Responses Induced by Chemotherapeutic Agents in Mice. Science (334): 1573. (2011)
- Jensen, M. & Riddell, S. Designing chimeric antigen receptors to effectively and safely target tumors. Current Opinion in Immunology. (33):9–15. (2015)
- Sadelain, M., Brentjens, R. & Rivière, I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discovery.
- Galluzzi, L. et al. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. (28): 690–714. (2015)
- Galluzzi, L. et al. The secret ally: immunostimulation by anticancer drugs. Nature Reviews Drug Discovery. (11): 215-233. (2012)
- Melief, C. et al. Therapeutic cancer vaccines. The Journal of Clinical Investigation. 125(9): 3401–3412. (2015)
- Palucka, K. et al. Dendritic-Cell-Based Therapeutic Cancer Vaccines. Immunity. (39): 38-48. (2013)
- Palucka, K. & Coussens, L. The Basis of Oncoimmunology. Cell. (164): 1233 – 1247.(2016)
- Rabinovich, G. & Croci, D. Regulatory Circuits Mediated by Lectin-Glycan Interactions in Autoimmunity and Cancer. Immunity. (36): 322-335. (2012)










