Introduction
- HIV is a retrovirus and made of double stranded RNA enclosed within a glycosylated capsid
- Target cells for HIV are any cell expressing both CD4 and CCR5 or CXCR4 (chemokine receptors)
- HIV has evolved to use these receptors to infect the “central command” of the immune system and by so doing ultimately disables the immune response
- Infection is persistent and chronic and there is only one known case of someone being cured of HIV infection
- The prevalence of HIV (numbers of people infected) in sub-Sahara Africa vary from 6-30%, with southern Africa bearing the brunt of infections
- Household surveys in South Africa have shown that on average approximately 15% of people are infected, with women between the ages of 22-26 years with the highest prevalence
- Antenatal testing shows that around 29% of pregnant women are infected
- With the advent of antiretroviral treatment in the public sector, mother to child transmission of HIV has been reduced to between 1-5% of babies born to HIV infected mothers
- HIV prevalence in males appears to peak at a later age than females, at around 30-35 year olds
- Understanding immunity to HIV is important for devising potential vaccine strategies as well as appreciating immunopathogenesis
Stages of HIV Infection
- Routes of HIV infection are predominantly through mucosal surfaces: male and female genital tracts, rectal surfaces and gut surfaces (perinatal infection)
- Acquisition of HIV can also be directly through the bloodstream from injection drug users
- Whatever the route of infection, there has been a defined stage of infection based on laboratory diagnosis
- The tools for measuring HIV infection in a diagnostic laboratory can be found within Immunopaedia here
- Figure 1 shows Fiebig staging of laboratory testing for HIV infection.
- Fiebig staging is a 6-stage classification system that was formulated for staging early HIV infection based on the different times viral markers and host antibody responses emerge.
- The system was named after the paper’s first author
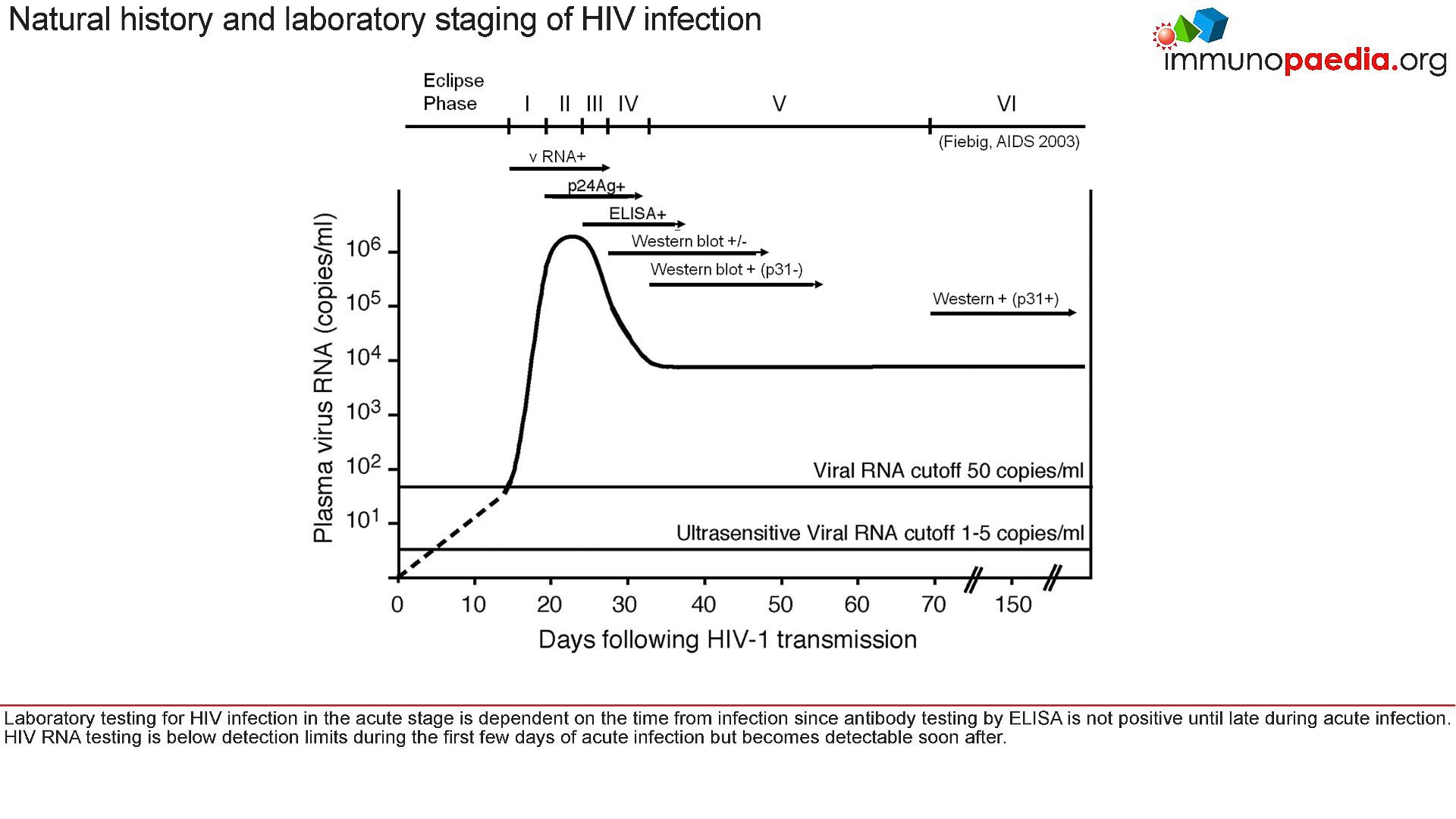
Figure 1: Fiebig staging of acute HIV infection. [Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003 Sep 5;17(13):1871-9]
- This description is based on the Case Study: A Case of Fever and General Malaise
- For example, at the acute stage of HIV infection (Fiebig I/II), which is prior to seroconversion and when there was peak viraemia, it has been shown that there is a cytokine storm
- This is where a number of proinflammatory cytokine levels are high, giving rise to the fever, pharyngitis, lymphadenopathy and
- As the peak viral load equilibriates to a set point, the ELISA test shows the presence of anti-p24 antibodies and the production of large amounts of viral proteins. These are detected in western blots.
- The viral set point is usually established around 3-6 months after initial infection
- The set point is where the level of HIV replication in the host is relatively constant over time
- It is thought that this represents an equilibrium between host immunity to HIV and the rate of viral turn over
- What is important to realise is that HIV is predominantly found in lymphoid tissue and not in the blood circulation
- However, it is known that the higher the viral load in the blood plasma, the faster disease progression occurs
- Conversely, the lower the viral load, the slower disease progresses
- In some individuals, HIV infection appears to be under control, and it is these individuals where biomarkers of immune control may be the most informative
The immune response to HIV
- There appears to be an ordered series of events that occur upon HIV acquisition
- Figure 2 shows a typical immune response to untreated HIV infection.
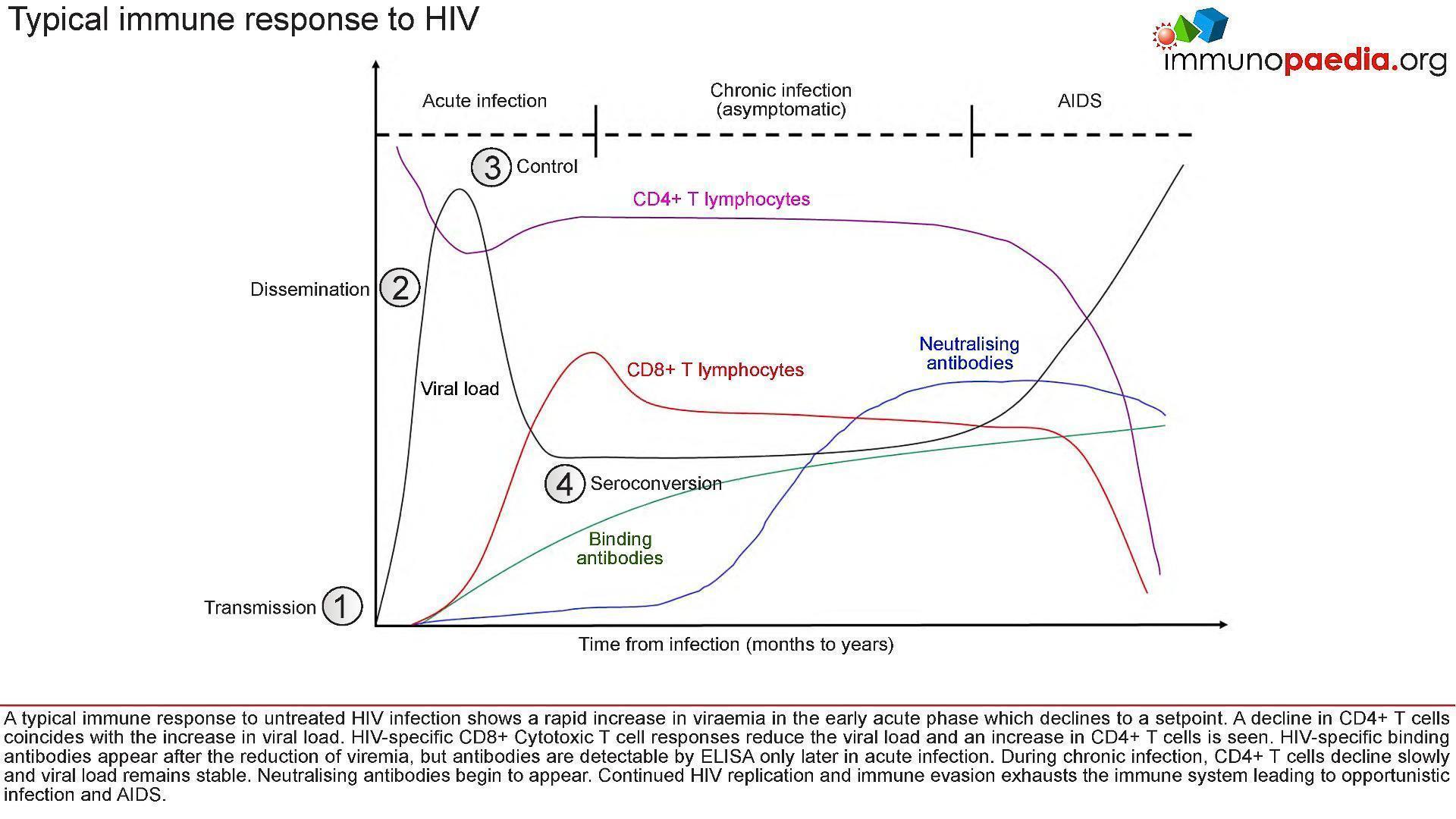
Figure 2: Stages of HIV infection from transmission to seroconversion and the immune events which occur at each stage.
- After transmission of HIV to a new host:
- (1), there is dissemination of the virus to lymphoid tissues
- (2) a rapid increase in viraemia in the acute phase (measured as Fiebig stage I). The fall in peak viraemia is thought to be due to the initial immune control
- (3) and viral load declines to a set point.
- A decline in CD4+ T cells coincides with the increase in viral load.
- HIV-specific CD8+ Cytotoxic T cell responses are thought to reduce systemic viral load and an increase in CD4+ T cells is often observed.
- HIV-specific binding antibodies appear after the reduction of viraemia, but antibodies are detectable by ELISA only later in acute infection (4, Fiebig stage III onwards).
- During chronic infection, CD4+ T cells decline slowly and viral load remains relatively stable.
- Neutralising antibodies begin to appear only after about 3-6 months and continued HIV replication
- Immune evasion exhausts the immune system leading to opportunistic infection and AIDS.
Let’s look at each of these four stages in closer detail:
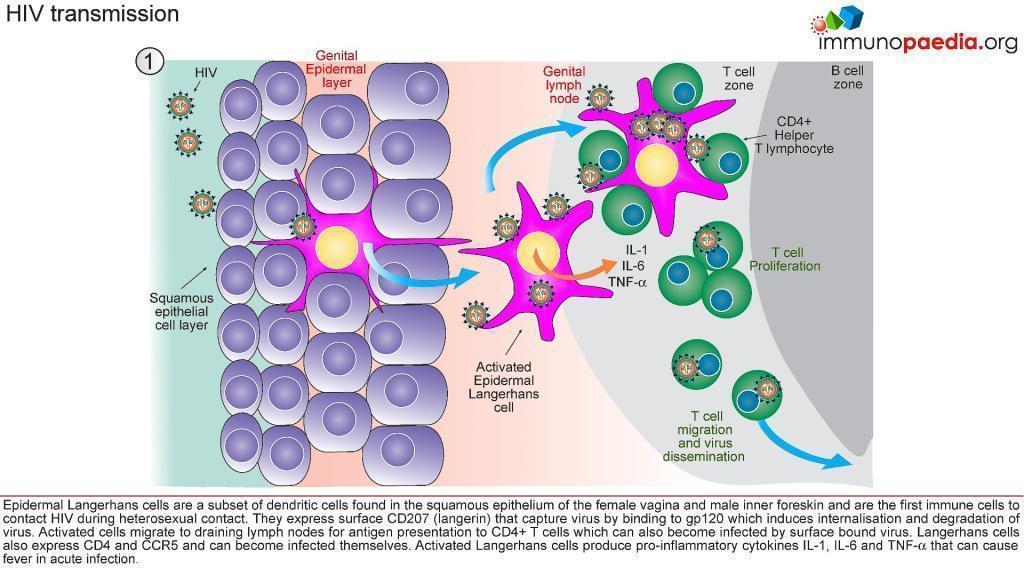
1) HIV Transmission.
- Infection is a “rare” event.
- In 80% of cases, transmission is thought to be established by a single virus
- All microorganisms that penetrate the epithelial surfaces are met immediately by cells and molecules that can mount an innate immune response (Figure 3)
- Epidermal Langerhans’ cells are a subset of dendritic cells found in the squamous epithelium of the female vagina and male inner foreskin and are the first immune cells to contact HIV during heterosexual contact.
- They express surface CD207 (langerin) that captures virus by binding to gp120, which induces internalisation and degradation of virus particles.
- Activated Langerhans’ cells migrate to draining lymph nodes for antigen presentation to CD4+ and CD8+ T cells.
- In the process, CD4+ T cells can also become infected by virus bound to the Langerhans cell surface (trans-infection).
- Langerhans’ cells may also express CD4 and CCR5 and can become infected themselves.
- Activated Langerhans’ cells produce pro-inflammatory cytokines IL-1, IL-6 and TNF-α that can cause fever.
- Dilation and increased permeability of the blood vessels during inflammation leads to increased local blood flow
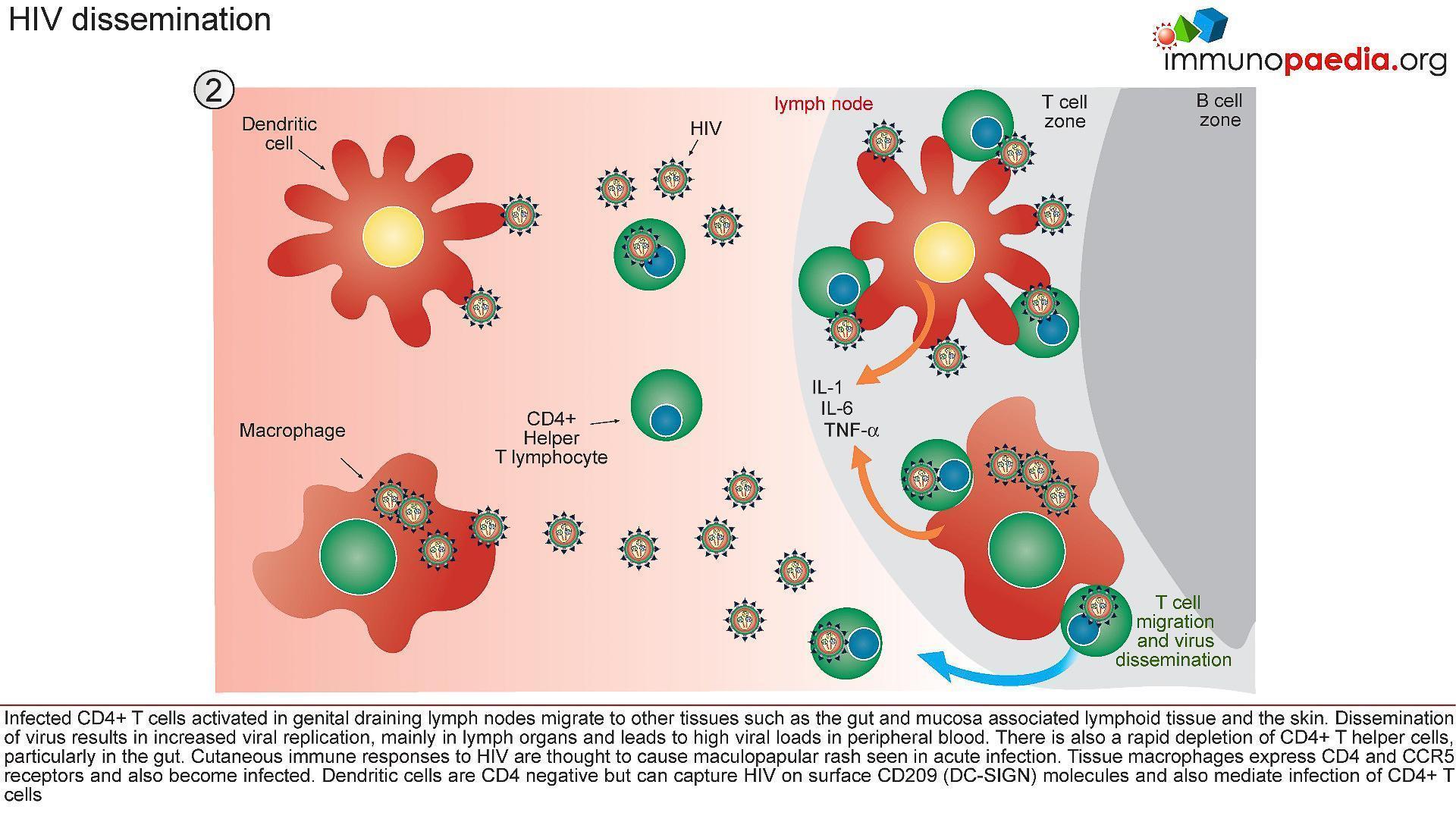
2) HIV Dissemination
- Afferent lymphatic vessels drain fluid from the tissues and carry antigen bearing cells from infected tissues to the lymph nodes where they are trapped (Figure 4)
- Follicles expand as B lymphocytes proliferate to form germinal centres and the entire lymph node enlarges (lymphadenopathy)
- HIV infected CD4+ T cells, activated in genital draining lymph nodes, migrate to mucosal tissues such as the gut and skin.
- Dissemination of virus results in increased viral replication, mainly in lymph organs and leads to high viral loads in peripheral blood.
- There is also a rapid depletion of CD4+ T cells, particularly in the gut lymphoid tissues.
- Tissue macrophages express CD4 and CCR5 receptors and also become infected.
- Dendritic cells are CD4 negative but can capture HIV on surface CD209 (DC-SIGN) molecules and mediate trans-infection of CCR5-bearing CD4+ T cells
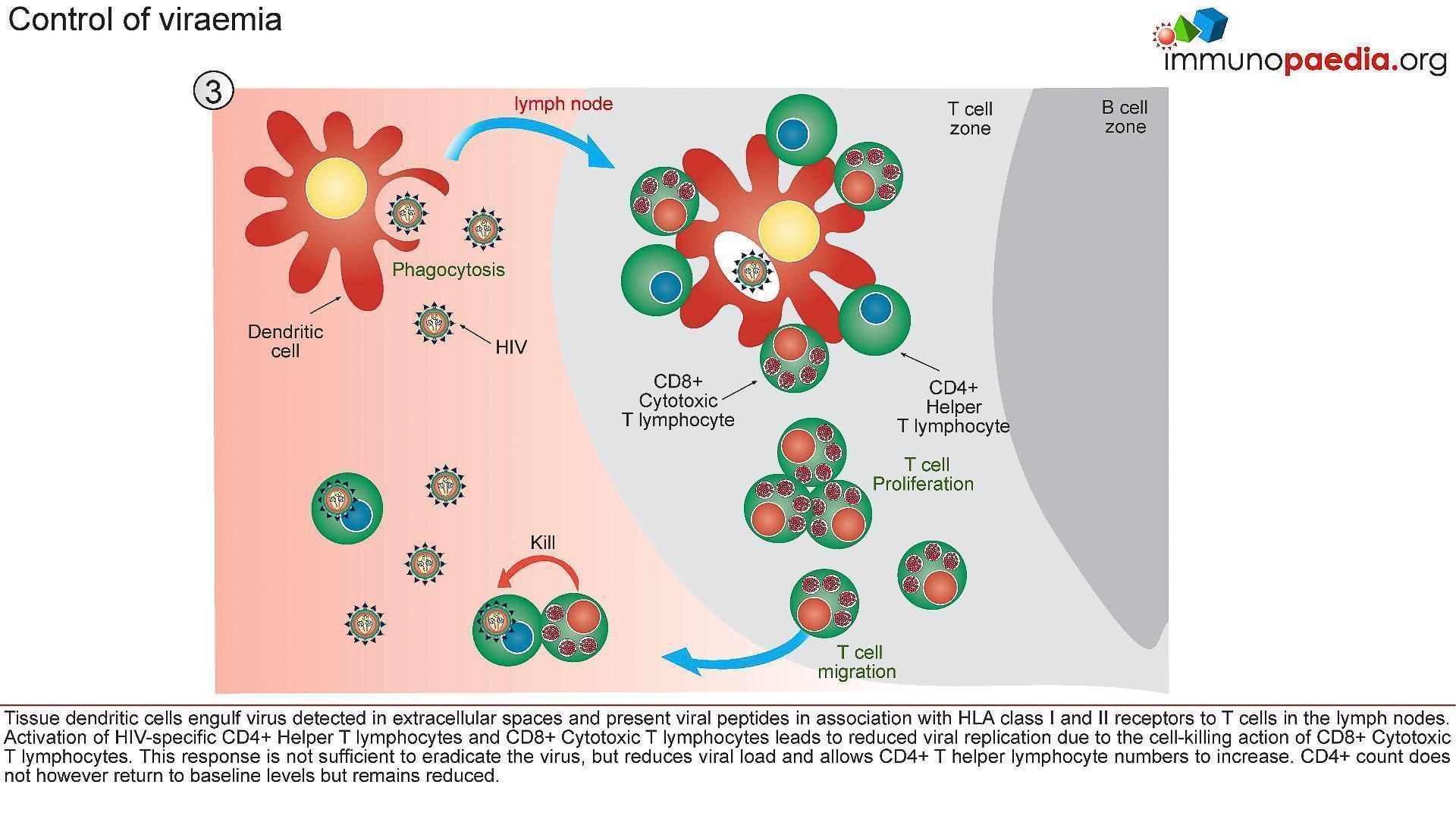
3) Control of Viraemia
- The partial resolution of peak viral load observed during the acute stage of HIV infection is associated with robust T cell immunity (Figure 5).
- Tissue dendritic cells engulf virus detected in extracellular spaces and present viral peptides by both HLA class I and II molecules in the lymph nodes to CD8+ and CD4+ T cells, respectively.
- Activated HIV-specific CD8+ cytotoxic T lymphocytes impart viral control by killing HIV infected cells and reducing viral replication.
- This response is not sufficient to eradicate the virus, but reduces viral load and allows CD4+ T helper lymphocyte numbers to increase.
- The absolute CD4+ count does not however return to baseline levels but remains reduced.
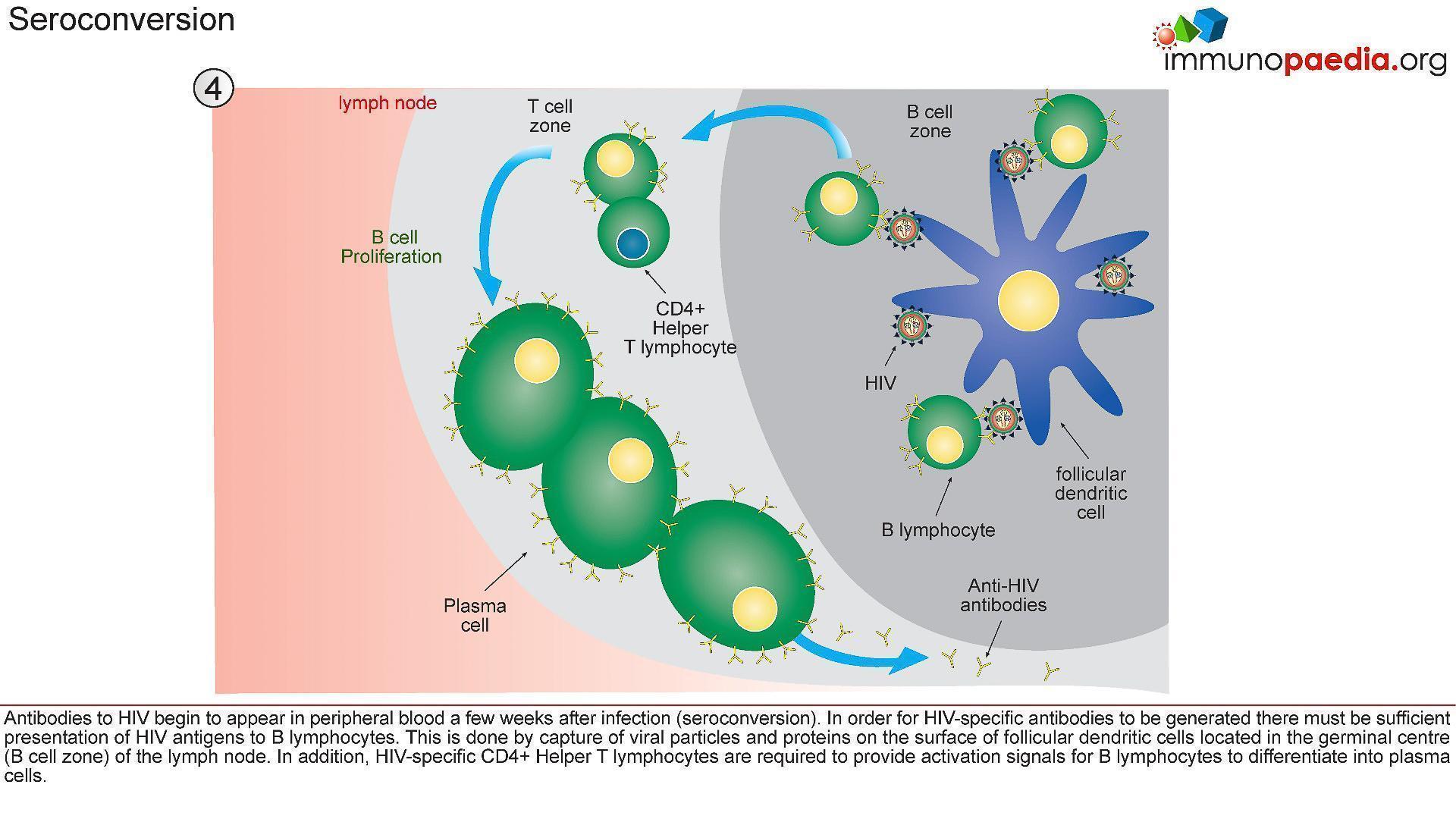
4) Seroconversion
- A multitude of immunological events have occured prior to seroconversion, many of them resulting in the clinical symptoms of acute retroviral syndrome.
- Antibodies to HIV (seroconversion) only begin to appear in peripheral blood 4-6 weeks after transmission, but in rare instances can take up to 3 months.
- In order for HIV-specific antibodies to be generated there must be sufficient presentation of HIV antigens to B lymphocytes (Figure 6)
- This is achieved by capture of viral particles and proteins on the surface of follicular dendritic cells located in the lymphoid follicles (B cell zone) of the lymph node.
- In addition, HIV-specific CD4+ helper T cells are required to provide activation signals for B cells to differentiate into plasma cells.
Quiz
Immunopaedia Case study
A case of fever and general malaise
Related Talk
Timothy Ray Brown, The Berlin Patient – The only known person to be cured of HIV
Wendy Burgers, University of Cape Town – Viruses and HIV