The human immunodeficiency virus (HIV), classified as a retrovirus, belongs to the genus Lentivirus within the family of Retroviridae. It is the causative agent of acquired immunodeficiency syndrome (AIDS). It invades various immune cells such as the CD4+ T cells and monocytes. A decline in CD4+ T cell numbers below the critical level leads to loss of cell-mediated immunity. Consequently, the body becomes progressively more susceptible to opportunistic infections and cancer.
HIV invasion of immune cells
- CCR5 and CXCR4 are expressed on the surface of host immune cells and act as coreceptors for HIV entry into human cells.
- HIV infects T cells via high-affinity interaction between the virion envelope glycoprotein (gp120) and the CD4 molecule.
- Infection of T cells is assisted by the T-cell co-receptor, CXCR4 while HIV infects monocytes by interacting with CCR5 co-receptor (Figure 1). During the early stages of HIV infection, viral isolates tend to use CCR5 for viral entry, while later isolates tend to use CXCR4.
- As shown in Figure 2, after gp120 binds to CD4 on the T cell (1), nucleocapsids containing viral genome and enzymes enters the target cell (2).
- Following the release of viral genome and enzymes from the core protein, viral reverse transcriptase catalyses reverse transcription of ssRNA to form RNA-DNA hybrids (3).
- The viral RNA template is partially degraded by ribonuclease H and the second DNA strand is synthesized (4).
- The viral dsDNA is translocated into the nucleus and integrated into the host genome by the viral integrase enzyme (5).
- Transcription factors transcribe the proviral DNA into genomic ssRNA (6), which is exported to cytoplasm (7).
- In the cytoplasm, host-cell ribosomes catalyse synthesis of viral precursor proteins (8).
- The viral precursor proteins are cleaved into viral proteins by viral proteases (9).
- HIV ssRNA and proteins assemble beneath the host-cell plasma membrane (10) forming virion buds from it (11).
- Maturation occurs either in the forming buds or after budding from the host cell (12).
- During maturation, HIV proteases cleave the poly-proteins into individual functional HIV proteins.
- The mature virions are able to infect another host cell.
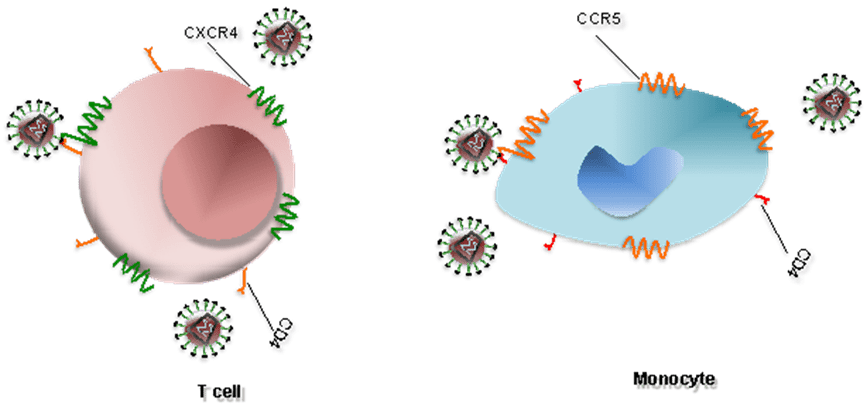
Figure 1. Interaction between HIV and coreceptors of a T cell and a monocyte [Alouani, N. and Berrezouga, P. L. (2021) “Oral HPV and HIV: A case report with analysis of the recent literature.” Unpublished. doi: 10.13140/RG.2.2.15621.17120.]
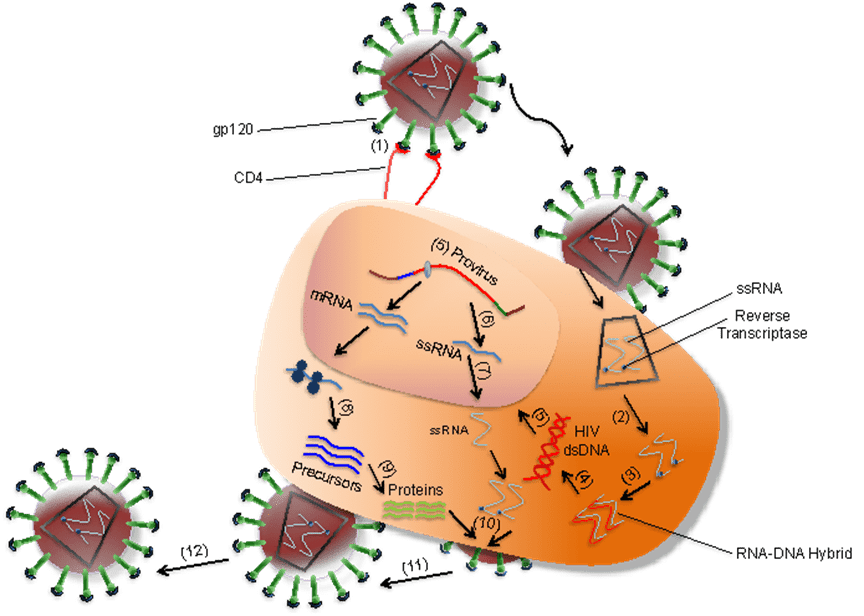
Figure 2. Overview of HIV infection of a target cell (e.g. T cell) [Alouani, N. and Berrezouga, P. L. (2021) “Oral HPV and HIV: A case report with analysis of the recent literature.” Unpublished. doi: 10.13140/RG.2.2.15621.17120.]
Innate immune response to HIV
- Innate immune cells (e.g., dendritic cells and natural killer cells) are the first line of defense which HIV encounters upon entry to the body.
Macrophages
- Tissue macrophages are one of the target cells for HIV.
- They harbour the virus and are known to be the source of viral proteins.
Dendritic cells (DCs)
- DCs are large cells with dendritic cytoplasmic extensions.
- These cells present processed antigens to T lymphocytes in lymph nodes.
- Epidermal DCs, are probably among the first immune cells to combat HIV at the mucosal surfaces.
- These cells transport HIV from the site of infection to lymphoid tissue.
- The follicular DCs, found in lymphoid tissue, are also key antigen-presenting cells that trap and present antigens on their cell surfaces.
- In the lymph node follicles, DCs provide signals for the activation of B lymphocytes.
Natural killer (NK) cells.
- NK cells have lytic activity against cells that have diminished expression of major histocompatibility complex (MHC) I
- Because the presence of MHC class I is required for peptide presentation to T cell receptors, NK cells are an important line of defense when HIV escapes the cellular immune response.
- NK cells proliferate in response to type 1 interferon secreted by DCs.
- These stimulated NK cells release cytokines such as interferon γ (IFN-γ), tumour necrosis factor α (TNF-α), and chemokines to activate T-cell proliferation (cellular immune response).
- NK cells also inhibit viral replication by releasing IFN-γ.
Adaptive immune response to HIV
Cellular immune response to HIV
- This is induced upon the entry of HIV into the target cells (e.g., T cells) and synthesis of viral proteins (Figure 1).
- MHC class I on the cell surface displays the intracellularly degraded HIV peptide fragments for recognition by T-cell receptors (TCR) on CD8+ T cells (Figure 3).
- CD8+ T cells lyse HIV infected cells and secrete cytokines, i.e. interferon-γ (IFN-γ), tumor necrosis factor α (TNF-α), and chemokines, i.e. MIP-1 α, MIP β and RANTES, that inhibit virus replication and block viral entry into CD4+ T cells.
- Development of CD8+ T cells is crucial for control of HIV replication.
- This results in declining viraemia after primary infection. In the early stages of infection, CD4+ T cells lose their proliferative capacity and therefore their contribution to viral control is minor. However, during chronic infection CD4+T cells are present and secrete interleukin-2(IL-2) or cytokines, such as IFN-γ, to control viraemia.
Humoral response to HIV
- Occurs later in infection (Figure 4); therefore, the level of antibodies during the acute infection is very low.
- Non-neutralising antibodies to structural proteins (i.e. P17 and P24) are first to appear and generally do not persist.
- Later, neutralising antibodies specific to proteins involved in the entry of the virus into the cells, will be generated.
- These antibodies are specific to: (1) the variable region of gp120 (V3); (2) CD4 binding sites and chemokine receptors (i.e., CXCR4 and CCR5); (3) the transmembrane protein gp41.
- Potent neutralizing antibodies have been shown to play a major role in controlling HIV infection in a few symptom-free HIV+ individuals who maintain high level of CD4+ T cells and low viral load.
A summary of the Immune Response to HIV is available as a wallchart – https://www.stemcell.com/media/files/wallchart/10000011477-Immune_Response_HIV.pdf
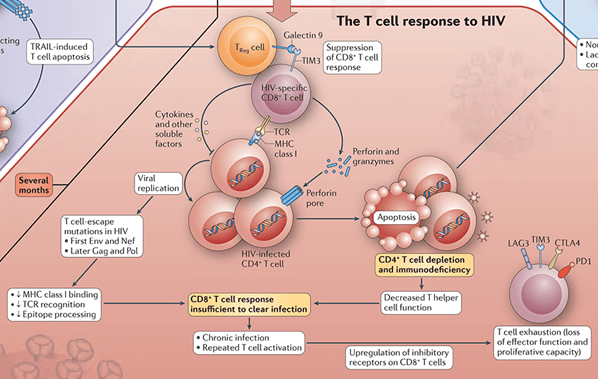
Figure 3: The T Cell Response to HIV [Adapted from the Immune Response to HIV wallchart, created by Nature Reviews Immunology in partnership with STEMCELL Technologies. Stemcell.com. https://www.stemcell.com/immunology-features/immune-response-to-hiv (Accessed: Oct 25, 2022)]
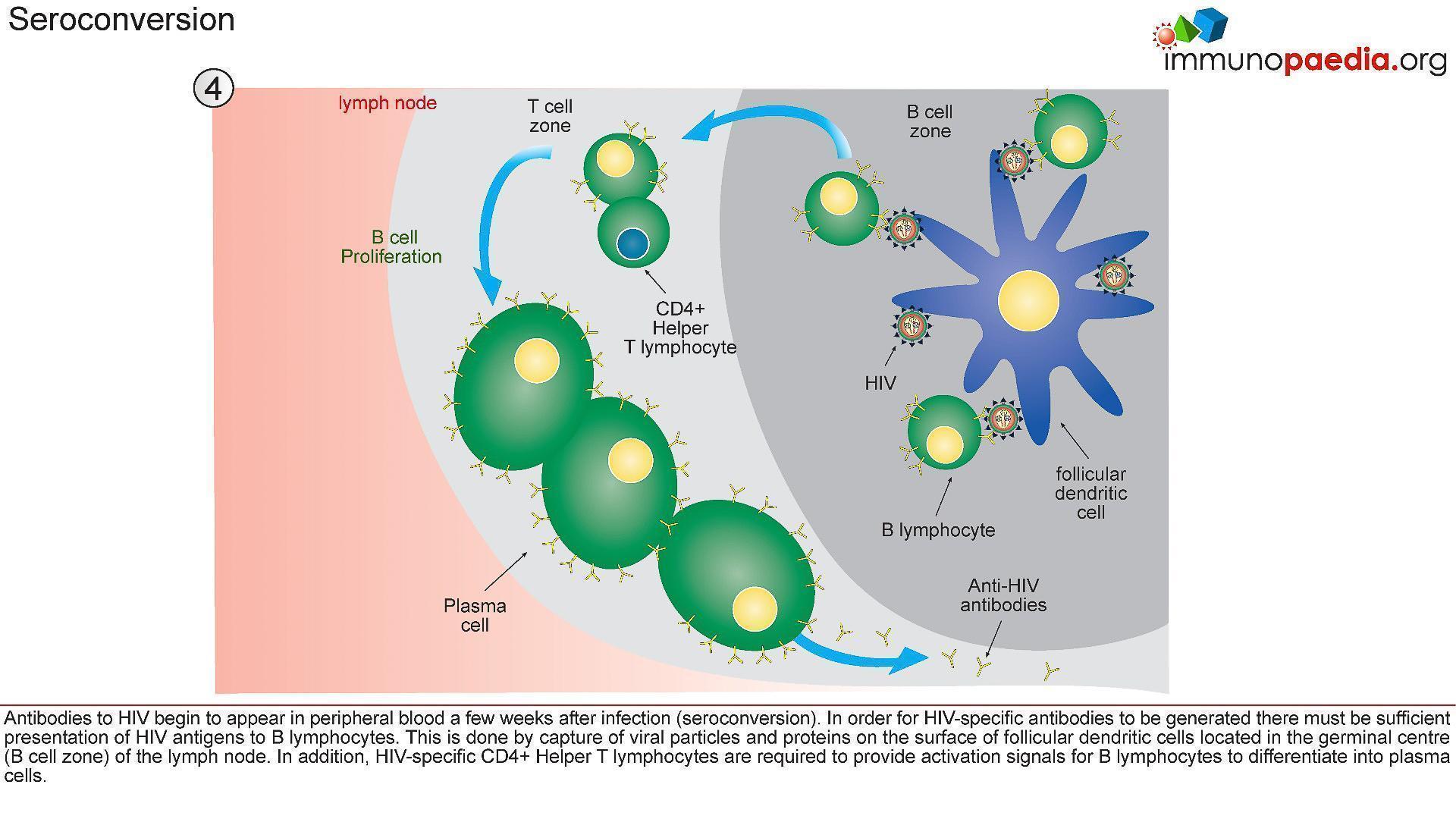
Figure 4: Immune events leading to antibody production and seroconversion. [Shaan (2016) Immunity to HIV, Immunopaedia | Advancing global immunology education. Immunopaedia]
Insights into HIV vaccine design efforts
- The development of an effective HIV-1 vaccine is particularly challenging owing to the exceptional and increasing genetic diversity of the HIV-1 lentivirus. Some of this results from its complex mechanisms of immune evasion and the ability of HIV-1 to integrate into host immune cells to become resistant to host immunity and treatment regimens.
- The first generation of vaccines tested in clinical trials utilized gp120 as an antigen for eliciting neutralizing antibodies, whereas more recent trials tested vaccines designed to elicit CD8+ T cell responses and non-neutralizing antibodies.
- Out of eight HIV-1 vaccine efficacy trials completed so far, only one trial showed that high levels of antibodies binding to the HIV-1 variable loop 2 (V2) and low levels of IgA specific for the envelope (Env) protein correlated with decreased transmission and guided the design of subsequent clinical trials.
- However, of two phase IIb/III clinical trials designed to improve on the RV144 trial — HIV-1 Vaccine Trials Network (HVTN) 702 (NCT02968849) and HVTN 705 (NCT03060629) — neither showed significant efficacy, suggesting that the RV144 trial may not have been a harbinger of vaccine success.
- An ongoing phase III trial is further exploring the concept of inducing high titres of non-neutralizing antibodies (HVTN 706, NCT03964415)
- Thus, for HIV-1, the induction of antibodies that broadly protect against heterologous HIV-1 strains (called broadly neutralizing antibodies (bnAbs) is a prime goal of HIV-1 vaccine development
HIV and Cancer
People infected with HIV have a substantially higher risk of some types of cancers compared to uninfected people of the same age. The general term for these cancers is “HIV-associated cancers.”
Three different viruses are known to be causes of these cancers known as “acquired immunodeficiency syndrome (AIDs)-defining cancers” or “AIDS-defining malignancies”;
- Kaposi sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), which causes Kaposi sarcoma and some subtypes of lymphoma
- Epstein-Barr virus (EBV), which causes some subtypes of non-Hodgkin and Hodgkin lymphoma
- Human papillomaviruses (HPV), which cause cervical cancer, most anal cancers, and oropharyngeal, penile, vaginal, and vulvar cancer
Other viruses that are most likely to cause cancer in people with HIV are Hepatitis B virus (HBV) and hepatitis C virus (HCV), which both cause liver cancer. A diagnosis of any of these aforementioned cancers in someone infected with HIV confirms a diagnosis of AIDS.
Infection with HIV weakens the immune system and reduces the body’s ability to fight viral infections that may lead to cancer. Also, because people infected with HIV have compromised immune systems, both immunosuppression and inflammation may have direct or indirect roles in the development of some cancers that are elevated in people infected with HIV.
The poorer cancer survival of HIV-infected people may result, at least in part, from the weakened immune system in such individuals. HIV infection is therefore associated with an increased risk of dying from cancer.
The introduction of highly active antiretroviral therapy (HAART), also called combination antiretroviral therapy (cART), starting in the mid-1990s, greatly reduced the incidence of certain cancers in HIV-infected patients, especially Kaposi’s sarcoma and non-Hodgkin’s lymphoma.
Although the risk of these AIDS-defining cancers among people infected with HIV is lower than in the past, it is still much higher than what it seen in the general population. This persistently high risk may reflect the fact that cART does not completely restore immune system functioning.
Quiz
Download these Resources:
References
- Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis.Lancet 2007; 370(9581):59–67.
- Hernández-Ramírez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study.Lancet HIV 2017 Aug 10. pii: S2352-3018(17)30125-X.
- Wang CC, Silverberg MJ, Abrams DI. Non-AIDS-defining malignancies in the HIV-infected population.Current Infectious Disease Reports 2014; 16(6):406.
- Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative incidence of cancer among persons with HIV in North America: A cohort study.Annals of Internal Medicine 2015; 163(7):507-518.
- Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States.Journal of Clinical Oncology 2015; 33(21):2376-2383.
- Coghill AE, Pfeiffer RM, Shiels MS, Engels EA. Excess mortality among HIV-infected individuals with cancer in the United States.Cancer Epidemiology, Biomarkers & Prevention 2017 Jun 15. doi: 10.1158/1055-9965.EPI-16-0964.
- Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals.Journal of Acquired Immune Deficiency Syndromes2009; 52(5):611-22.
- Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention.Current Opinion in Oncology 2012; 24(5):506-16
- Goncalves PH, Montezuma-Rusca JM, Yarchoan R, Uldrick TS. Cancer prevention in HIV-infected populations.Semininars in Oncology 2016; 43(1):173-188.
- Powles T, Macdonald D, Nelson M, Stebbing J. Hepatocellular cancer in HIV-infected individuals: tomorrow’s problem?Expert Review of Anticancer Therapy 2006; 6(11):1553–1558.
- Angeletti PC, Zhang L, Wood C.The viral etiology of AIDS-associated malignancies. Advances in Pharmacology 2008; 56:509–557.
- Silverberg MJ, Abrams DI. AIDS-defining and non-AIDS-defining malignancies: cancer occurrence in the antiretroviral therapy era.Current Opinion in Oncology 2007; 19(5):446–451.
- Grogg KL, Miller RF, Dogan A. HIV infection and lymphoma.Journal of Clinical Pathology 2007; 60(12):1365–1372.
- Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis.AIDS2016; 30(2):273-291.
- Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States.AIDS 2014; 28(6):881–890.
- Macdonald DC, Nelson M, Bower M, Powles T. Hepatocellular carcinoma, human immunodeficiency virus and viral hepatitis in the HAART era.World Journal of Gastroenterology 2008; 14(11):1657–1663.
- McGinnis KA, Fultz SL, Skanderson M, et al. Hepatocellular carcinoma and non-Hodgkin’s lymphoma: the roles of HIV, hepatitis C infection, and alcohol abuse.Journal of Clinical Oncology 2006; 24(31):5005–5009.
- Naggie S, Cooper C, Saag M, et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1.New England Journal of Medicine 2015; 373(8):705-713.
- Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1.New England Journal of Medicine 2015; 373(8):714-725.
- Robbins HA, Strickler HD, Massad LS, et al. Cervical cancer screening intervals and management for women living with HIV: a risk benchmarking approach.AIDS 2017; 31(7):1035-1044.
- Goldie SJ, Kuntz KM, Weinstein MC, et al. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men.Journal of the American Medical Association 1999; 281(19):1822–1829.
- Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis.Lancet 2007; 370(9581):59–67.
- Hernández-Ramírez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study.Lancet HIV 2017 Aug 10. pii: S2352-3018(17)30125-X.
- Wang CC, Silverberg MJ, Abrams DI. Non-AIDS-defining malignancies in the HIV-infected population.Current Infectious Disease Reports 2014; 16(6):406.
- Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative incidence of cancer among persons with HIV in North America: A cohort study.Annals of Internal Medicine 2015; 163(7):507-518.
- Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated cancer-specific mortality among HIV-infected patients in the United States.Journal of Clinical Oncology 2015; 33(21):2376-2383.
- Coghill AE, Pfeiffer RM, Shiels MS, Engels EA. Excess mortality among HIV-infected individuals with cancer in the United States.Cancer Epidemiology, Biomarkers & Prevention 2017 Jun 15. doi: 10.1158/1055-9965.EPI-16-0964.
- Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals.Journal of Acquired Immune Deficiency Syndromes2009; 52(5):611-22.
- Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention.Current Opinion in Oncology 2012; 24(5):506-16
- Goncalves PH, Montezuma-Rusca JM, Yarchoan R, Uldrick TS. Cancer prevention in HIV-infected populations.Semininars in Oncology 2016; 43(1):173-188.
- Powles T, Macdonald D, Nelson M, Stebbing J. Hepatocellular cancer in HIV-infected individuals: tomorrow’s problem?Expert Review of Anticancer Therapy 2006; 6(11):1553–1558.
- Angeletti PC, Zhang L, Wood C.The viral etiology of AIDS-associated malignancies. Advances in Pharmacology 2008; 56:509–557.
- Silverberg MJ, Abrams DI. AIDS-defining and non-AIDS-defining malignancies: cancer occurrence in the antiretroviral therapy era.Current Opinion in Oncology 2007; 19(5):446–451.
- Grogg KL, Miller RF, Dogan A. HIV infection and lymphoma.Journal of Clinical Pathology 2007; 60(12):1365–1372.
- Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis.AIDS2016; 30(2):273-291.
- Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States.AIDS 2014; 28(6):881–890.
- Macdonald DC, Nelson M, Bower M, Powles T. Hepatocellular carcinoma, human immunodeficiency virus and viral hepatitis in the HAART era.World Journal of Gastroenterology 2008; 14(11):1657–1663.
- McGinnis KA, Fultz SL, Skanderson M, et al. Hepatocellular carcinoma and non-Hodgkin’s lymphoma: the roles of HIV, hepatitis C infection, and alcohol abuse.Journal of Clinical Oncology 2006; 24(31):5005–5009.
- Naggie S, Cooper C, Saag M, et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1.New England Journal of Medicine 2015; 373(8):705-713.
- Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1.New England Journal of Medicine 2015; 373(8):714-725.
- Robbins HA, Strickler HD, Massad LS, et al. Cervical cancer screening intervals and management for women living with HIV: a risk benchmarking approach.AIDS 2017; 31(7):1035-1044.
- Goldie SJ, Kuntz KM, Weinstein MC, et al. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men.Journal of the American Medical Association 1999; 281(19):1822–1829.










