There have been many advances in HIV care and treatment over the past 40 years. Despite these advances, there is no cure for HIV nor a preventive HIV vaccine. This article summarises the recent IUIS-Immunopaedia-Frontiers in Immunology webinar featuring Professor Lynn Morris biomedical scientist* at the National Institute for Communicable Diseases (NICD) South Africa who focused on HIV prevention: antibodies and vaccine development.
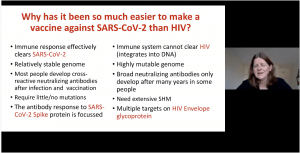 Prof. Lynn Morris began her talk comparing the HIV and SARS-Cov-2 epidemics, at the peak of the HIV epidemic approximately annual mortality was 1.8 million deaths reported due to HIV/AIDS (similar COVID-19 mortality observed n 2020). Fortunately, HIV associated mortality has drastically decreased due to the successful roll-out of anti-retroviral treatment. Prof. Morris highlighted amazing strides the COVID-19 vaccinology field has made, including the development of eight vaccines that were approved for (emergency) use. This is in stark contrast to the HIV vaccine field, where only seven efficacy trials have taken place in the last 20 years, none of which have been approved for use. Prof Morris then discussed differences in immune responses associated with COVID-19 and HIV, which enabled the development of effective vaccines against COVID-19 but poses challenges to HIV vaccinology. Prof Morris also highlighted the following trials
Prof. Lynn Morris began her talk comparing the HIV and SARS-Cov-2 epidemics, at the peak of the HIV epidemic approximately annual mortality was 1.8 million deaths reported due to HIV/AIDS (similar COVID-19 mortality observed n 2020). Fortunately, HIV associated mortality has drastically decreased due to the successful roll-out of anti-retroviral treatment. Prof. Morris highlighted amazing strides the COVID-19 vaccinology field has made, including the development of eight vaccines that were approved for (emergency) use. This is in stark contrast to the HIV vaccine field, where only seven efficacy trials have taken place in the last 20 years, none of which have been approved for use. Prof Morris then discussed differences in immune responses associated with COVID-19 and HIV, which enabled the development of effective vaccines against COVID-19 but poses challenges to HIV vaccinology. Prof Morris also highlighted the following trials
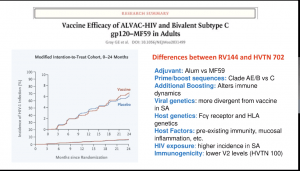 (i) RV144 (ALVAC. And AIDSVAC vaccine) trial that demonstrated a 31% efficacy against the acquisition of infection and demonstrated that non-neutralising Abs correlated against the reduced risk of infection
(i) RV144 (ALVAC. And AIDSVAC vaccine) trial that demonstrated a 31% efficacy against the acquisition of infection and demonstrated that non-neutralising Abs correlated against the reduced risk of infection- (ii) HVTN702 trial (similar vaccine as the one tested in the RV144 trials, however, vaccines were modified to clades that are dominant in Sub-Saharan Africa) which was, unfortunately, stopped early due to similar infection rates in vaccine and placebo group.
- (iii) HVTN705/706 efficacy trial of Ad26.Mos4.HV and Clade C gp140 HIV vaccines were designed to induce non-neutralising Abs and showed good protection in non-human primates (results expected in 2022/3)
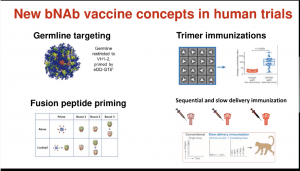
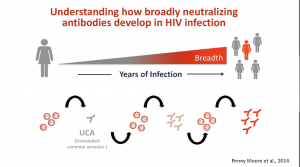 The ultimate aim of the HIV vaccine field is to induce broadly neutralising Abs (bNAbs). Unfortunately, bNAbs take years to develop and may not happen in all individuals, thus a key focus is to understand immunological processes associated with bNAb development with the hope of inducing a similar response using vaccination. New bNAb vaccine concepts are currently in phase 1 clinical trial testing and include
The ultimate aim of the HIV vaccine field is to induce broadly neutralising Abs (bNAbs). Unfortunately, bNAbs take years to develop and may not happen in all individuals, thus a key focus is to understand immunological processes associated with bNAb development with the hope of inducing a similar response using vaccination. New bNAb vaccine concepts are currently in phase 1 clinical trial testing and include
- (i) Germline Targeting approach is designed to stimulate rare B cells that give rise to Abs that bind the CD4 HIV binding site.
- (ii) Trimer Immunisations with soluble versions of the HIV glycoprotein.
- (iii) Fusion peptide priming
- (iv) Sequential and slow delivery immunisation priming and boosting immunity
 Prof Morris ended this section of her talk by highlighting how the Moderna vaccine was initially designed for HIV, and how the HIV field is investigating the potential for mRNA based HIV vaccines.
Prof Morris ended this section of her talk by highlighting how the Moderna vaccine was initially designed for HIV, and how the HIV field is investigating the potential for mRNA based HIV vaccines.
In the next summary, we shall highlight discussion points focused on passive immunisation and findings from the Antibody-Mediated Prevention (AMP) trial.
Summary by Cheleka AM Mpande
*Director of the Antibody Immunity Research Unit at the University of the Witwatersrand. Over the last 20 years Lynn has made significant contributions to understanding the antibody response to HIV infection and co-discovered the CAP256-VRC26.25 monoclonal antibody that is undergoing clinical testing for HIV prevention. Her laboratory performs neutralizing antibody assays for HIV vaccine trials and played a key role in the proof-of-concept Antibody Mediated Prevention (AMP) trial.










