Ebola virus (EBOV) is a negative-sense ssRNA virus belonging to the family Filoviridae of the order Mononegavirales, first described in 1976. The genus EBOV includes five known virus species: Bundibugyo ebolavirus, Reston ebolavirus, Sudan ebolavirus, Taï Forest ebolavirus (former Côte d’Ivoire ebolavirus), and Zaire ebolavirus. Human transmission of EBV is via bodily fluids and blood and causes severe and often fatal disease Ebola virus disease (EVD) with a 2-21 day incubation period. EVD can have diverse symptoms, like gastrointestinal symptoms or in severe cases( which are usually associated with high viral load) Ebola haemorrhagic fever (EHF). For more detail read Immunity to Ebola Course material.
EBOV infection causes widespread immune activation and is associated with perturbation of immune cells. In a recent IUIS-Immunopeadia-Frontiers of Immunology webinar, Anita McElroy* from the University of Pittsburgh Center for Vaccine Research) describes the cellular immune 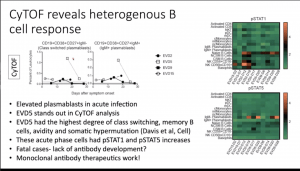 landscape that occurs following Ebola virus infection and will correlate these findings with key features of Ebola virus disease. Data presented was from 4 patients (3 moderate EVD, 1 severe EVD). To characterise immune responses associated with EVD, McElroy and colleagues used mass cytometry.
landscape that occurs following Ebola virus infection and will correlate these findings with key features of Ebola virus disease. Data presented was from 4 patients (3 moderate EVD, 1 severe EVD). To characterise immune responses associated with EVD, McElroy and colleagues used mass cytometry.
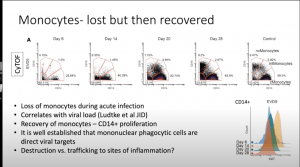
 McElroy presented data that demonstrated that severe disease and high viral load is associated with T cell activation, B cell expansion and monocyte redistribution as well as alterations in dendritic cells. These findings provide clues as to why EVD is so severe. Specific differences observed during EVD pathology compared to controls were:
McElroy presented data that demonstrated that severe disease and high viral load is associated with T cell activation, B cell expansion and monocyte redistribution as well as alterations in dendritic cells. These findings provide clues as to why EVD is so severe. Specific differences observed during EVD pathology compared to controls were:
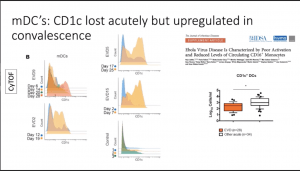
- Significant reduction of all monocyte populations (i.e. Classical, non-classical and intermediate monocytes) and myeloid-derived DCs (mDCs). These cell populations are quickly replenished (by day 28 post-symptom onset) upon recovery of EVD.
- Data from in vitro data by others have suggested that plasmacytoid DCs are note infected by EBOV and do not play a role in EVOD. However, data illustrated that pDCs are activated (high CD38 expression) during the acute phase of EVOD.
 Elevated levels of plasmablasts (IgM+ CD19+CD38+CD27+) and pSTAT1 and pSTAT5 expression by B cells.
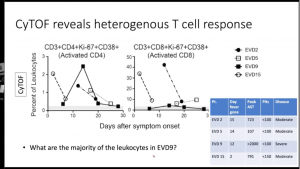
Elevated levels of plasmablasts (IgM+ CD19+CD38+CD27+) and pSTAT1 and pSTAT5 expression by B cells.- Despite multiple studies demonstrating a correlation between high levels of CD8 T cells activation and severe disease. Data presented showed similar levels of CD8 T cell (Ki-67+CD38+) activation level in severe EVD (n=1) and healthy controls, thus suggesting high levels of CD8 T cell activation is not characteristic of severe EVD immunopathology.s
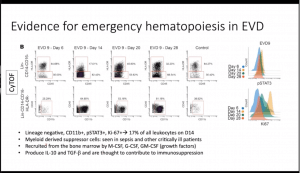
- Characterisation of other cells demonstrated an expansion of myeloid-derived suppressor cells (activation of emergency haematopoiesis) that produce IL-10 and TGFB (not investigated in mass cytometry data), a cell population associated with immuno-suppression
- Using unsupervised clustering they detected two cell populations (i) Lineage-CD11b+KI-67+pSTAT1+ and (ii) CD4+CD8+Ki67+pSTAT1+ which were significantly increased during diseases acute phase of infection and decreased to similar levels as health controls during recovery.
Summary by Cheleka AM Mpande