The Delgoffe Lab studies the metabolic contributions to T cell fate and function. The activation, expansion, and differentiation of T cells requires the interpretation of many complex signals including those from the antigen receptor, costimulatory and coinhibitory molecules, and cytokines. It has now become clear that metabolism represents another key mechanism by which T cells can be regulated. In addition, nutrient sensing and metabolic reprogramming pathways are intrinsically tied to signalling involved in T cell biology. Given that nutrients are limiting in the microenvironment of cancer, their studies have immediate translation as modalities to improve cancer immunotherapy.
Immunotherapy has changed how cancer is treated, although response rates remain low due in part to resistance mechanisms concentrated within the tumour microenvironment. In this seminar, Greg Delgoffe will discuss how the metabolic features of the tumour microenvironment can shape T cell differentiation to alternative fates and highlight how metabolic reprogramming can be harnessed to promote superior antitumor immunity.
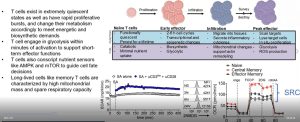
In the beginning of this talk, he highlights the importance and impact of immunotherapy in cancer treatment and how only 10% of patients receive beneficial results from this therapy which needs to be improved. The question then remains, why does this only work in about 10% of patients. This sparked interest in his lab where they are looking at the tumour microenvironment in order to look at how these therapies react at the site of tumours based on their constituents (Figure 1).
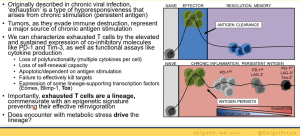
He further elaborates on tumour cell metabolism and how it effects this microenvironment and thus immunotherapy response. This is further exacerbated by the metabolic demands of T cells (as he explains, Figure 2). He highlights how T cells in tumours are defective and how they used mouse models to investigate this.
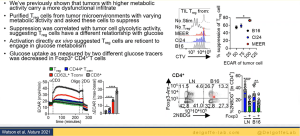
His lab went on to further investigate T cell exhaustion in terms of hypoxia (Figure 3). Using Pimonidazole, they were able to mimic hypoxia in mice to demonstrate how hypoxia plays a role in T cell exhaustion and therefore immunotherapy responses in individuals. They therefore hypothesised that hypoxia may alter the way that T cells respond to various signals. Using various experimental models, the team were able to describe, in detail, the relationship between T cell exhaustion and hypoxia. Interestingly, the team were able to show that not all T cell subsets are exhausted within these tumour microenvironments and regulatory T cells thrive within these environments (Figure 4). They were able to describe this using a mouse suppression model.
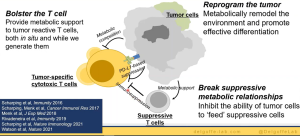
Finally, Dr. Delgoffe highlighted new research that his team are doing looking at therapeutic T cells for immunotherapy i.e., growing several types of T cells in vitro and infuse them into patients undergoing treatment. However, they were looking to improve on existing methods used to grow T cells as the normal media used, X-VIVO 15, has a higher-than-normal glucose supplementation, leading to possible stress/failure when reinfused into patients as the level drops significantly (this could lead to T cell exhaustion).
In summary, Figure 5, he closed by highlighted the importance of understanding T cell function and metabolism and how we should lower the metabolic inhibitors for immunotherapy. The closing of this talk was followed by some interesting questions.
Summary by Stefan Botha